Ischemic Postconditioning (IPostC) Protects Fibrotic and Cirrhotic Rat Livers after Warm Ischemia
- PMID: 31281804
- PMCID: PMC6590494
- DOI: 10.1155/2019/5683479
Ischemic Postconditioning (IPostC) Protects Fibrotic and Cirrhotic Rat Livers after Warm Ischemia
Abstract
Background: Decreased organ function following liver resection is a major clinical issue. The practical method of ischemic postconditioning (IPostC) has been studied in heart diseases, but no data exist regarding fibrotic livers.
Aims: We aimed to determine whether IPostC could protect healthy, fibrotic, and cirrhotic livers from ischemia reperfusion injury (IRI).
Methods: Fibrosis was induced in male SD rats using bile duct ligation (BDL, 4 weeks), and cirrhosis was induced using thioacetamide (TAA, 18 weeks). Fibrosis and cirrhosis were histologically confirmed using HE and EvG staining. For healthy, fibrotic, and cirrhotic livers, isolated liver perfusion with 90 min of warm ischemia was performed in three groups (each with n=8): control, IPostC 8x20 sec, and IPostC 4x60 sec. additionally, healthy livers were investigated during a follow-up study. Lactate dehydrogenase (LDH) and thromboxane B2 (TXB2) in the perfusate, as well as bile flow (healthy/TAA) and portal perfusion pressure, were measured.
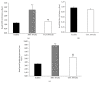
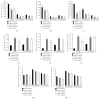
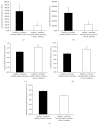
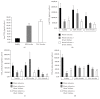
Results: LDH and TXB2 were reduced, and bile flow was increased by IPostC, mainly in total and in the late phase of reperfusion. The follow-up study showed that the perfusate derived from a postconditioned group had much less damaging potential than perfusate derived from the nonpostconditioned group.
Conclusion: IPostC following warm ischemia protects healthy, fibrotic, and cirrhotic livers against IRI. Reduced efflux of TXB2 is one possible mechanism for this effect of IPostC and increases sinusoidal microcirculation. These findings may help to improve organ function and recovery of patients after liver resection.
Figures


References
-
- Mendes-Braz M., Elias-Miró M., Jiménez-Castro M. B., Casillas-Ramírez A., Ramalho F. S., Peralta C. The current state of knowledge of Hepatic Ischemia-ReperfusionInjury Based on Its Study in Experimental Models. Journal of Biomedicine and Biotechnology. 2012;2012:20. doi: 10.1155/2012/298657.298657 - DOI - PMC - PubMed
-
- Abu-Amara M., Gurusamy K. S., Hori S., Glantzounis G., Fuller B., Davidson B. R. Pharmacological interventions for ischaemia reperfusion injury in liver resection surgery performed under vascular control. Cochrane Database of Systematic Reviews. 2009;4 doi: 10.1002/14651858.CD008154.CD007472 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

