Design, Synthesis, and Biological Evaluation of Novel Biotinylated Podophyllotoxin Derivatives as Potential Antitumor Agents
- PMID: 31281809
- PMCID: PMC6596340
- DOI: 10.3389/fchem.2019.00434
Design, Synthesis, and Biological Evaluation of Novel Biotinylated Podophyllotoxin Derivatives as Potential Antitumor Agents
Abstract
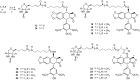
Podophyllotoxin has long been used as an active substance for cytotoxic activity. Fourteen novel biotinylated podophyllotoxin derivatives were designed, synthesized, and evaluated for cytotoxic activity for this study. The synthesized compounds were evaluated for cytotoxic activity in the following human cancer cell lines, SW480, MCF-7, A-549, SMMC-7721, and HL-60 by MTT assay. Most of them exhibited potent cytotoxic effects and compound 15 showed the highest cytotoxic activity among the five cancer cell lines tested, having its IC50 values in the range of 0.13 to 0.84 μM. Apoptosis analysis revealed that compound 15 caused obvious induction of cell apoptosis. Compound 15 significantly down-regulated the expression level of the marker proteins (caspase-3 and PARP) in H1299 and H1975 cells, activated the transcription of IRE1α, increased the expression of GRP78 and XBP-1s, and finally induced apoptosis of H1299 cells. In vivo studies showed that 15 at a dose of 20 mg/kg suppressed tumor growth of S180 cell xenografts in icr mice significantly. Further molecular docking studies suggested that compound 15 could bind well with the ATPase domain of Topoisomerase-II. These data suggest that compound 15 is a promising agent for cancer therapy deserving further research.
Keywords: anticancer activity; apoptosis; biotin; podophyllotoxin derivatives; synthesis.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

