High Thymic Output of Effector CD4+ Cells May Lead to a Treg : T Effector Imbalance in the Periphery in NOD Mice
- PMID: 31281853
- PMCID: PMC6594269
- DOI: 10.1155/2019/8785263
High Thymic Output of Effector CD4+ Cells May Lead to a Treg : T Effector Imbalance in the Periphery in NOD Mice
Abstract
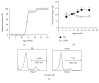
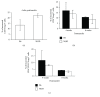
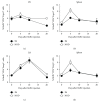
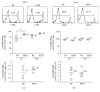
Regulatory T cells (Tregs) play a critical role in controlling autoreactive T cells, and quantitative and/or qualitative deficiencies in Tregs are associated with autoimmune diseases, including type 1 diabetes (T1D), in both humans and mice. Both the incidence of T1D and percentages of peripheral Tregs in NOD mice vary considerably between animal facilities. In our animal facility, the incidence of T1D in NOD mice is high at 90-100% and the percentages of peripheral CD4+Foxp3+ cells in ~9-10-week-old female NOD mice are decreased compared to control (B6) mice shortly before high glucose is first detected (~12 weeks). These data suggest that there is an imbalance between Tregs and potentially pathogenic effector T cells at this age that could have significant impact on disease progression to overt diabetes. The goal of the current study was to investigate mechanisms that play a role in peripheral Treg : T effector cell balance in NOD mice, including differences in persistence/survival, peripheral homeostatic proliferation, and thymic production and output of CD4+ T cells. We found no differences in persistence/survival or homeostatic proliferation of either Tregs or effector T cells between NOD and B6 mice. Furthermore, although the percentages and absolute numbers of CD4+Foxp3+ cells in thymus were not decreased in NOD compared to B6 mice, the percentage of CD4+ recent thymic emigrants (RTE) that were Foxp3+ was significantly lower in 9-week-old NOD mice. Interestingly, the thymic output of CD4+Foxp3+ cells was not lower in NOD mice, whereas the thymic output of CD4+Foxp3- cells was significantly higher in NOD mice at that age compared to B6 mice. These data suggest that the higher thymic output of CD4+Foxp3- T cells contributes, at least in part, to the lower percentages of peripheral CD4+Foxp3+ Tregs in NOD mice and an imbalance between Tregs and T effector cells that may contribute to the development of full-blown diabetes.
Figures



Similar articles
-
Thymically-derived Foxp3+ regulatory T cells are the primary regulators of type 1 diabetes in the non-obese diabetic mouse model.PLoS One. 2019 Oct 24;14(10):e0217728. doi: 10.1371/journal.pone.0217728. eCollection 2019. PLoS One. 2019. PMID: 31647813 Free PMC article.
-
Glucocorticoid hormone differentially modulates the in vitro expansion and cytokine profile of thymic and splenic Treg cells.Immunobiology. 2019 Mar;224(2):285-295. doi: 10.1016/j.imbio.2018.12.002. Epub 2018 Dec 27. Immunobiology. 2019. PMID: 30612787
-
Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes.Proc Natl Acad Sci U S A. 2007 Nov 13;104(46):18181-6. doi: 10.1073/pnas.0708899104. Epub 2007 Nov 8. Proc Natl Acad Sci U S A. 2007. PMID: 17991775 Free PMC article.
-
Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
-
Thymic function in HIV-infection.Dan Med J. 2013 Apr;60(4):B4622. Dan Med J. 2013. PMID: 23651726 Review.
Cited by
-
Anti-CD20 therapy ameliorates β cell function and rebalances Th17/Treg cells in NOD mice.Endocrine. 2022 Apr;76(1):44-52. doi: 10.1007/s12020-021-02965-x. Epub 2022 Jan 24. Endocrine. 2022. PMID: 35067899
References
-
- Alard P., Manirarora J. N., Parnell S. A., Hudkins J. L., Clark S. L., Kosiewicz M. M. Deficiency in NOD antigen-presenting cell function may be responsible for suboptimal CD4+CD25+ T-cell-mediated regulation and type 1 diabetes development in NOD mice. Diabetes. 2006;55(7):2098–2105. doi: 10.2337/db05-0810. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

