Effect of Mass Artesunate-Amodiaquine Distribution on Mortality of Patients With Ebola Virus Disease During West African Outbreak
- PMID: 31281856
- PMCID: PMC6602760
- DOI: 10.1093/ofid/ofz250
Effect of Mass Artesunate-Amodiaquine Distribution on Mortality of Patients With Ebola Virus Disease During West African Outbreak
Abstract
Background: Experiments in vitro have shown that the drug amodiaquine may inhibit Ebola virus activity. During the Ebola virus disease (EVD) epidemic in West Africa in 2014-2016, 2 mass drug administrations (MDAs) of artesunate-amodiaquine (ASAQ) were implemented to decrease the burden of malaria. The objective of this study was to assess the effect of the ASAQ MDAs on the mortality of patients with EVD.
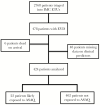
Methods: A retrospective cohort design was used to analyze mortality data for patients with EVD admitted to 5 Ebola treatment units in Liberia and Sierra Leone. Patients admitted to the ETUs during the time period of ASAQ's therapeutic effect from areas where the MDA was implemented were matched to controls not exposed to ASAQ, using a range of covariates, including malaria co-infection status, and a logistic regression analysis was performed. The primary outcome was Ebola treatment unit mortality.
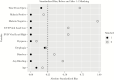
Results: A total of 424 patients with EVD had sufficient data for analysis. Overall, the mortality of EVD patients was 57.5%. A total of 22 EVD patients were exposed to ASAQ during the MDAs and were found to have decreased risk of death compared with those not exposed in a matched analysis, but this did not reach statistical significance (relative risk, 0.63; 95% confidence interval, 0.37-1.07; P = .086).
Conclusions: There was a non-statistically significantly decreased risk of mortality in EVD patients exposed to ASAQ during the 2 MDAs as compared with EVD patients not exposed to ASAQ. Further prospective trials are needed to determine the direct effect of ASAQ on EVD mortality.
Keywords: Ebola virus disease; amodiaquine; epidemic; mass drug administration; mortality.
Figures
References
-
- World Health Organization. Ebola situation report - 30 March 2016.2016. Available at: http://apps.who.int/ebola/current-situation/ebola-situation-report-30-ma.... Accessed September 1, 2018.
-
- World Health Organization. WHO supports Ebola vaccination of high risk populations in the Democratic Republic of the Congo 2018. Available at: http://www.who.int/news-room/detail/21-05-2018-who-supports-ebola-vaccin.... Accessed September 3, 2018.
-
- World Health Organization. Ebola situation reports: Democratic Republic of the Congo. 2018. Available at: http://www.who.int/ebola/situation-reports/drc-2018/en/. Accessed September 1, 2018.