Imaging CAR T cell therapy with PSMA-targeted positron emission tomography
- PMID: 31281894
- PMCID: PMC6609218
- DOI: 10.1126/sciadv.aaw5096
Imaging CAR T cell therapy with PSMA-targeted positron emission tomography
Abstract
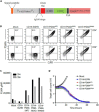
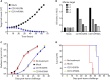
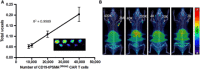
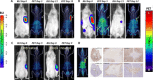
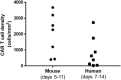
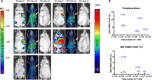
Chimeric antigen receptor (CAR) T cell therapy for hematologic malignancies is fraught with several unknowns, including number of functional T cells that engage target tumor, durability and subsequent expansion and contraction of that engagement, and whether toxicity can be managed. Non-invasive, serial imaging of CAR T cell therapy using a reporter transgene can address those issues quantitatively. We have transduced anti-CD19 CAR T cells with the prostate-specific membrane antigen (PSMA) because it is a human protein with restricted normal tissue expression and has an expanding array of positron emission tomography (PET) and therapeutic radioligands. We demonstrate that CD19-tPSMA(N9del) CAR T cells can be tracked with [18F]DCFPyL PET in a Nalm6 model of acute lymphoblastic leukemia. Divergence between the number of CD19-tPSMA(N9del) CAR T cells in peripheral blood and bone marrow and those in tumor was evident. These findings underscore the need for non-invasive repeatable monitoring of CAR T cell disposition clinically.
Figures
Comment in
-
Repurposing PSMA PET to Image CAR T Cell Immunotherapy.Radiol Imaging Cancer. 2019 Sep 27;1(1):e194004. doi: 10.1148/rycan.2019194004. eCollection 2019 Sep. Radiol Imaging Cancer. 2019. PMID: 33778676 Free PMC article. No abstract available.
-
Combination of Chemoembolization plus Radiofrequency Ablation May Provide Better Survival Outcomes than Monotherapy for HCC.Radiol Imaging Cancer. 2019 Sep 27;1(1):e194005. doi: 10.1148/rycan.2019194005. eCollection 2019 Sep. Radiol Imaging Cancer. 2019. PMID: 33778677 Free PMC article. No abstract available.
References
-
- Kochenderfer J. N., Wilson W. H., Janik J. E., Dudley M. E., Stetler-Stevenson M., Feldman S. A., Maric I., Raffeld M., Nathan D.-A., Lanier B. J., Morgan R. A., Rosenberg S. A., Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). - PMC - PubMed
-
- Brentjens R. J., Davila M. L., Riviere I., Park J., Wang X., Cowell L. G., Bartido S., Stefanski J., Taylor C., Olszewska M., Borquez-Ojeda O., Qu J., Wasielewska T., He Q., Bernal Y., Rijo I. V., Hedvat C., Kobos R., Curran K., Steinherz P., Jurcic J., Rosenblat T., Maslak P., Frattini M., Sadelain M., CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra38 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

