Monitoring of immunoglobulin N- and O-glycosylation in health and disease
- PMID: 31281930
- PMCID: PMC7225405
- DOI: 10.1093/glycob/cwz048
Monitoring of immunoglobulin N- and O-glycosylation in health and disease
Abstract
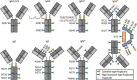
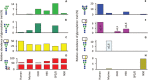
Protein N- and O-glycosylation are well known co- and post-translational modifications of immunoglobulins. Antibody glycosylation on the Fab and Fc portion is known to influence antigen binding and effector functions, respectively. To study associations between antibody glycosylation profiles and (patho) physiological states as well as antibody functionality, advanced technologies and methods are required. In-depth structural characterization of antibody glycosylation usually relies on the separation and tandem mass spectrometric (MS) analysis of released glycans. Protein- and site-specific information, on the other hand, may be obtained by the MS analysis of glycopeptides. With the development of high-resolution mass spectrometers, antibody glycosylation analysis at the intact or middle-up level has gained more interest, providing an integrated view of different post-translational modifications (including glycosylation). Alongside the in-depth methods, there is also great interest in robust, high-throughput techniques for routine glycosylation profiling in biopharma and clinical laboratories. With an emphasis on IgG Fc glycosylation, several highly robust separation-based techniques are employed for this purpose. In this review, we describe recent advances in MS methods, separation techniques and orthogonal approaches for the characterization of immunoglobulin glycosylation in different settings. We put emphasis on the current status and expected developments of antibody glycosylation analysis in biomedical, biopharmaceutical and clinical research.
Keywords: antibody; biopharmaceutical; glycan; glycoproteomics; mass spectrometry.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Abrahams JL, Campbell MP, Packer NH. 2018. Building a PGC-LC-MS N-glycan retention library and elution mapping resource. Glycoconj J. 35:15–29. - PubMed
-
- Arnaud J, Audfray A, Imberty A. 2013. Binding sugars: From natural lectins to synthetic receptors and engineered neolectins. Chem Soc Rev. 42:4798–4813. - PubMed
-
- Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. 2007. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 25:21–50. - PubMed
-
- Bobaly B, D'Atri V, Lauber M, Beck A, Guillarme D, Fekete S. 2018. Characterizing various monoclonal antibodies with milder reversed phase chromatography conditions. J Chromatogr B Analyt Technol Biomed Life Sci. 1096:1–10. - PubMed

