Effectiveness of a Bundled Intervention Including Adjunctive Corticosteroids on Outcomes of Hospitalized Patients With Community-Acquired Pneumonia: A Stepped-Wedge Randomized Clinical Trial
- PMID: 31282921
- PMCID: PMC6618815
- DOI: 10.1001/jamainternmed.2019.1438
Effectiveness of a Bundled Intervention Including Adjunctive Corticosteroids on Outcomes of Hospitalized Patients With Community-Acquired Pneumonia: A Stepped-Wedge Randomized Clinical Trial
Abstract
Importance: Community-acquired pneumonia remains a leading cause of hospitalization, mortality, and health care costs worldwide. Randomized clinical trials support the use of adjunctive corticosteroids, early progressive mobilization, antibiotic switching rules, and dietary interventions in improving outcomes. However, it is uncertain whether implementing these interventions will translate into effectiveness under routine health care conditions.
Objective: To evaluate the effectiveness of a bundle of evidence-supported treatments under conditions of routine care in a representative population hospitalized for community-acquired pneumonia.
Design, setting, and participants: A double-blind, stepped-wedge, cluster-randomized clinical trial with 90-day follow-up was conducted between August 1, 2016, and October 29, 2017, in the general internal medicine service at 2 tertiary hospitals in Melbourne, Australia, among a consecutive sample of patients with community-acquired pneumonia. The primary analysis and preparation of results took place between May 14 and November 25, 2018.
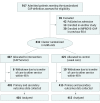
Interventions: Treating clinical teams were advised to prescribe prednisolone acetate, 50 mg/d, for 7 days (in the absence of any contraindication) and de-escalate from parenteral to oral antibiotics according to standardized criteria. Algorithm-guided early mobilization and malnutrition screening and treatment were also implemented.
Main outcomes and measures: Hospital length of stay, mortality, readmission, and intervention-associated adverse events (eg, gastrointestinal bleeding and hyperglycemia).
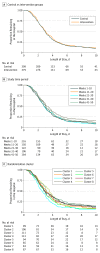
Results: A total of 917 patients were screened, and 816 (351 women and 465 men; mean [SD] age, 76 [13] years) were included in the intention-to-treat analysis, with 401 patients receiving the intervention and 415 patients in the control group. An unadjusted geometric mean ratio of 0.95 (95% CI, 0.78-1.16) was observed for the difference in length of stay (days) between the intervention and control groups. Similarly, no significant differences were observed for the secondary outcomes of mortality and readmission, and the results remained unchanged after further adjustment for sex and age. The study reported higher proportions of gastrointestinal bleeding in the intervention group (9 [2.2%]) compared with the controls (3 [0.7%]), with an unadjusted estimated difference in mean proportions of 0.008 (95% CI, 0.005-0.010).
Conclusions and relevance: This bundled intervention including adjunctive corticosteroids demonstrated no evidence of effectiveness and resulted in a higher incidence of gastrointestinal bleeding. Efficacy of individual interventions demonstrated in clinical trials may not necessarily translate into effectiveness when implemented in combination and may even result in net harm.
Trial registration: ClinicalTrials.gov identifier: NCT02835040.
Conflict of interest statement
Figures
Comment in
-
In inpatients with community-acquired pneumonia, a bundled intervention with steroids did not reduce length of stay.Ann Intern Med. 2019 Nov 19;171(10):JC52. doi: 10.7326/ACPJ201911190-052. Ann Intern Med. 2019. PMID: 31739335 No abstract available.
References
-
- Institute for Health Metrics and Evaluation GBD Compare website. https://vizhub.healthdata.org/gbd-compare/. Accessed September 27, 2018.
-
- Australian Institute of Health and Welfare Separation statistics by principal diagnosis (ICD-10-AM, 8th Edition), Australia, 2013-14 to 2014-15. https://www.aihw.gov.au/reports/hospitals/principal-diagnosis-data-cubes.... Accessed March 7, 2017.
Associated data
LinkOut - more resources
Full Text Sources
Medical

