Regioselective Functionalization of [2.2]Paracyclophanes: Recent Synthetic Progress and Perspectives
- PMID: 31283092
- PMCID: PMC7003812
- DOI: 10.1002/anie.201904863
Regioselective Functionalization of [2.2]Paracyclophanes: Recent Synthetic Progress and Perspectives
Abstract
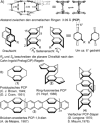
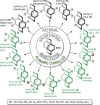
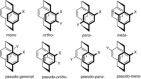
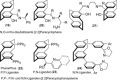
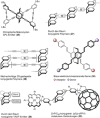
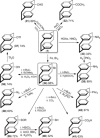
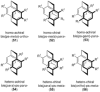
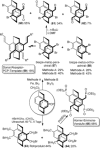
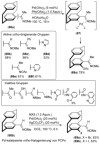
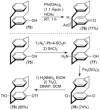
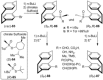
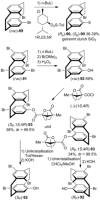
[2.2]Paracyclophane (PCP) is a prevalent scaffold that is widely utilized in asymmetric synthesis, π-stacked polymers, energy materials, and functional parylene coatings that finds broad applications in bio- and materials science. In the last few years, [2.2]paracyclophane chemistry has progressed tremendously, enabling the fine-tuning of its structural and functional properties. This Minireview highlights the most important recent synthetic developments in the selective functionalization of PCP that govern distinct features of planar chirality as well as chiroptical and optoelectronic properties. Special focus is given to the function-inspired design of [2.2]paracyclophane-based π-stacked conjugated materials by transition-metal-catalyzed cross-coupling reactions. Current synthetic challenges, limitations, as well as future research directions and new avenues for advancing cyclophane chemistry are also summarized.
Keywords: [2.2]paracyclophanes; chirality; functional parylenes; regioselective synthesis; π-stacked polymers.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



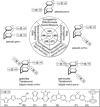
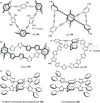

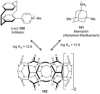
References
-
- Brown C. J., Farthing A. C., Nature 1949, 164, 915–916. - PubMed
-
- Cram D. J., Steinberg H., J. Am. Chem. Soc. 1951, 73, 5691–5704.
-
- Cram D. J., Cram J. M., Acc. Chem. Res. 1971, 4, 204–213.
-
- Modern Cyclophane Chemistry (Eds.: R. Gleiter, H. Hopf), Wiley-VCH, Weinheim, 2004.
-
- Robertson J. M., Organic Crystals and Molecules, Cornell University Press, Ithaca, 1953, p. 213.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

