Living With Neuroendocrine Tumors: Assessment of Quality of Life Through a Mobile Application
- PMID: 31283354
- PMCID: PMC6873920
- DOI: 10.1200/CCI.19.00025
Living With Neuroendocrine Tumors: Assessment of Quality of Life Through a Mobile Application
Abstract
Purpose: To understand the quality of life (QoL) for patients with neuroendocrine tumors (NETs) through comparison of QoL questionnaires and symptom tracking as well as journaling via the Carcinoid NETs Health Storylines mobile application (app).
Patients and methods: This was a 12-week prospective, observational study of US patients with NET who were taking long-acting somatostatin analogs. National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) and European Organisation for Research and Treatment of Cancer (EORTC) questionnaires were administered three times. Patients also monitored symptoms, mood, bowel movements, food, activity, and sleep, and they journaled in their app, which was coded by theme and sentiment for qualitative analysis.
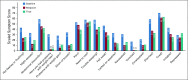
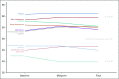
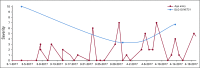
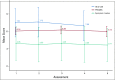
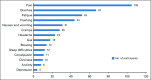
Results: Of the 120 patients with NET, 78% were women (mean age, 57 years); 76% had gastroenteropancreatic NETs, and 88% had metastases. Lanreotide depot and octreotide long-acting release (LAR) were used by 41% and 59%, respectively. The most common symptoms at baseline were fatigue (76.7%), diarrhea (62.5%), abdominal discomfort (64.1%), and trouble sleeping (57.5%). The majority completed five of six survey assessments (median, 5; mean, 5.1) and tracked four symptoms in the app (median, 4; mean, 5.5); the average frequency was 41.6 days for each symptom (median, 43; mean, 41.6; range, 1 to 84 days [12 weeks]). Without treatment change, most EORTC-assessed physical symptoms decreased from baseline to midpoint (eg, 59.3% at baseline v 33% at midpoint reported "quite a bit" or "very much" diarrhea; P = .002). App-based symptom tracking revealed large day-to-day variation, but weekly averages correlated well with survey scores. Journal entries showed that more patients made predominantly negative unsolicited entries about their injection experience with octreotide LAR compared with lanreotide (13 of 17 v two of 13; P < .001).
Conclusion: Patients with NET experience a large symptom burden that varies daily. A decrease in physical symptoms on QoL surveys suggests an effect from daily app-based monitoring or journaling, which may reduce recall bias and benefit the patient's experience of symptoms.
Conflict of interest statement
Jared R. Adams
David Ray
Renee Willmon
Sonia Pulgar
Arvind Dasari
No other potential conflicts of interest were reported.
Figures
References
-
- Pearman TP, Beaumont JL, Cella D, et al. Health-related quality of life in patients with neuroendocrine tumors: An investigation of treatment type, disease status, and symptom burden. Support Care Cancer. 2016;24:3695–3703. - PubMed
-
- Coon CD. The use of patient-reported outcomes in demonstrating safety and efficacy in oncology. Clin Ther. 2016;38:756–758. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

