In Vivo Imaging-Driven Approaches to Study Virus Dissemination and Pathogenesis
- PMID: 31283440
- PMCID: PMC7217087
- DOI: 10.1146/annurev-virology-101416-041429
In Vivo Imaging-Driven Approaches to Study Virus Dissemination and Pathogenesis
Abstract
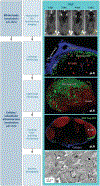
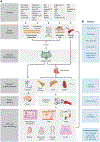
Viruses are causative agents for many diseases and infect all living organisms on the planet. Development of effective therapies has relied on our ability to isolate and culture viruses in vitro, allowing mechanistic studies and strategic interventions. While this reductionist approach is necessary, testing the relevance of in vitro findings often takes a very long time. New developments in imaging technologies are transforming our experimental approach where viral pathogenesis can be studied in vivo at multiple spatial and temporal resolutions. Here, we outline a vision of a top-down approach using noninvasive whole-body imaging as a guide for in-depth characterization of key tissues, physiologically relevant cell types, and pathways of spread to elucidate mechanisms of virus spread and pathogenesis. Tool development toward imaging of infectious diseases is expected to transform clinical diagnosis and treatment.
Keywords: immune response; noninvasive imaging; virus dissemination; virus infection.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

