What Are the Applications and Limitations of Artificial Intelligence for Fracture Detection and Classification in Orthopaedic Trauma Imaging? A Systematic Review
- PMID: 31283727
- PMCID: PMC6903838
- DOI: 10.1097/CORR.0000000000000848
What Are the Applications and Limitations of Artificial Intelligence for Fracture Detection and Classification in Orthopaedic Trauma Imaging? A Systematic Review
Abstract
Background: Artificial-intelligence algorithms derive rules and patterns from large amounts of data to calculate the probabilities of various outcomes using new sets of similar data. In medicine, artificial intelligence (AI) has been applied primarily to image-recognition diagnostic tasks and evaluating the probabilities of particular outcomes after treatment. However, the performance and limitations of AI in the automated detection and classification of fractures has not been examined comprehensively.
Question/purposes: In this systematic review, we asked (1) What is the proportion of correctly detected or classified fractures and the area under the receiving operating characteristic (AUC) curve of AI fracture detection and classification models? (2) What is the performance of AI in this setting compared with the performance of human examiners?
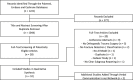
Methods: The PubMed, Embase, and Cochrane databases were systematically searched from the start of each respective database until September 6, 2018, using terms related to "fracture", "artificial intelligence", and "detection, prediction, or evaluation." Of 1221 identified studies, we retained 10 studies: eight studies involved fracture detection (ankle, hand, hip, spine, wrist, and ulna), one addressed fracture classification (diaphyseal femur), and one addressed both fracture detection and classification (proximal humerus). We registered the review before data collection (PROSPERO: CRD42018110167) and used the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). We reported the range of the accuracy and AUC for the performance of the predicted fracture detection and/or classification task. An AUC of 1.0 would indicate perfect prediction, whereas 0.5 would indicate a prediction is no better than a flip-of-a-coin. We conducted quality assessment using a seven-item checklist based on a modified methodologic index for nonrandomized studies instrument (MINORS).
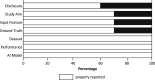
Results: For fracture detection, the AUC in five studies reflected near perfect prediction (range, 0.95-1.0), and the accuracy in seven studies ranged from 83% to 98%. For fracture classification, the AUC was 0.94 in one study, and the accuracy in two studies ranged from 77% to 90%. In two studies AI outperformed human examiners for detecting and classifying hip and proximal humerus fractures, and one study showed equivalent performance for detecting wrist, hand and ankle fractures.
Conclusions: Preliminary experience with fracture detection and classification using AI shows promising performance. AI may enhance processing and communicating probabilistic tasks in medicine, including orthopaedic surgery. At present, inadequate reference standard assignments to train and test AI is the biggest hurdle before integration into clinical workflow. The next step will be to apply AI to more challenging diagnostic and therapeutic scenarios when there is absence of certitude. Future studies should also seek to address legal regulation and better determine feasibility of implementation in clinical practice.
Level of evidence: Level II, diagnostic study.
Conflict of interest statement
All ICMJE Conflict of Interest Forms for authors and
Figures

Comment in
-
CORR Insights®: What Are the Applications and Limitations of Artificial Intelligence for Fracture Detection and Classification in Orthopaedic Trauma Imaging? A Systematic Review.Clin Orthop Relat Res. 2019 Nov;477(11):2492-2494. doi: 10.1097/CORR.0000000000000912. Clin Orthop Relat Res. 2019. PMID: 31369435 Free PMC article. No abstract available.
References
-
- Al-Helo S, Alomari RS, Ghosh S, Chaudhary V, Dhillon G, Al-Zoubi MB, Hiary H, Hamtini TM. Compression fracture diagnosis in lumbar: a clinical CAD system. Int J Comput Assist Radiol Surg. 2013;8:461-469. - PubMed
-
- Basha CMAKZ, Padmaja M, Balaji GN. Computer Aided Fracture Detection System. J Med Imaging Health Inform. 2018;8:526-531.
-
- Bayram F, Çakiroğlu M. DIFFRACT: DIaphyseal Femur FRActure Classifier SysTem. Biocybern Biomed Eng. 2016;36:157-171.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

