Inositol in preterm infants at risk for or having respiratory distress syndrome
- PMID: 31283839
- PMCID: PMC6613728
- DOI: 10.1002/14651858.CD000366.pub4
Inositol in preterm infants at risk for or having respiratory distress syndrome
Abstract
Background: Inositol is an essential nutrient required by human cells in culture for growth and survival. Inositol promotes maturation of several components of surfactant and may play a critical role in fetal and early neonatal life. A drop in inositol levels in infants with respiratory distress syndrome (RDS) can be a sign that their illness will be severe.
Objectives: To assess the effectiveness and safety of supplementary inositol in preterm infants with or without respiratory distress syndrome (RDS) in reducing adverse neonatal outcomes including: death (neonatal and infant deaths), bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC) and sepsis.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 11), MEDLINE via PubMed (1966 to 5 November 2018), Embase (1980 to 5 November 2018), and CINAHL (1982 to 5 November 2018). We searched clinical trial databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials (RCT) and quasi-randomised trials.
Selection criteria: We included all randomised controlled trials of inositol supplementation of preterm infants compared with a control group that received a placebo or no intervention. Outcomes included neonatal death, infant death, bronchopulmonary dysplasia (BPD), retinopathy of prematurity (ROP), intraventricular haemorrhage (IVH), necrotizing enterocolitis (NEC) and sepsis.
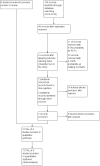
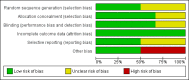
Data collection and analysis: The three review authors independently abstracted data on neonatal outcomes and resolved any disagreements through discussion and consensus. Outcomes were reported as typical risk ratio (RR), risk difference (RD) and number needed to treat for an additional beneficial outcome (NNTB) or number needed to treat for an additional harmful outcome (NNTH). We used the GRADE approach to assess the quality of evidence.
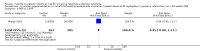
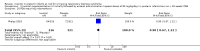
Main results: Six published randomised controlled trials were identified, with a total of 1177 infants. Study quality varied for the comparison 'Inositol supplementation to preterm infants (repeat doses in any amount and any duration of treatment) versus control' and interim analyses had occurred in several trials for the outcomes of interest. In this comparison, neonatal death was found to be significantly reduced (typical RR 0.53, 95% CI 0.31 to 0.91; typical RD -0.09, 95% CI -0.16 to -0.01; NNTB 11, 95% CI 6 to 100; 3 trials, 355 neonates). Infant deaths were not reduced (typical RR 0.89, 95% CI 0.71 to 1.13; typical RD -0.02, 95% CI -0.07 to 0.02; 5 trials, 1115 infants) (low-quality evidence). ROP stage 2 or higher or stage 3 or higher was not significantly reduced (typical RR 0.89, 95% CI 0.75 to 1.06; typical RD -0.04, 95% CI -0.10 to 0.02; 3 trials, 810 infants) (moderate-quality evidence). There were no significant findings for ROP (any stage), NEC (suspected or proven), sepsis, IVH grade greater than II (moderate-quality evidence). For the comparison 'Inositol supplementation IV initially followed by enteral administration (repeat doses of 80 mg/kg/day) in preterm infants born at less than 30 weeks' postmenstrual age (PMA) compared to placebo for preterm infants at risk for or having respiratory distress syndrome' the results from two studies of high quality were included (N = 760 neonates). Recruitment to the larger study (N = 638) was terminated because of a higher rate of deaths in the inositol group. We did not downgrade the quality of the study. The meta-analyses of the outcomes of 'Type 1 ROP or death before determination of ROP outcome using the adjudicated ROP outcome', 'Type 1 ROP including adjudicated ROP outcome', 'All-cause mortality (outcome collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)' and 'Severe IVH (grade 3 or 4)' did not show significant findings (moderate-quality evidence). There were no significant findings for the outcomes 'BPD or death by it prior to 37 weeks' postmenstrual age (outcomes collected through first event: death, hospital discharge, hospital transfer, or 120 days after birth)', 'Late onset sepsis (> 72 hours of age)', and 'Suspected or proven NEC' (high-quality evidence).
Authors' conclusions: Based on the evidence from randomised controlled trials to date, inositol supplementation does not result in important reductions in the rates of infant deaths, ROP stage 3 or higher, type 1 ROP, IVH grades 3 or 4, BPD, NEC, or sepsis. These conclusions are based mainly on two recent randomised controlled trials in neonates less than 30 weeks' postmenstrual age (N = 760), the most vulnerable population. Currently inositol supplementation should not be routinely instituted as part of the nutritional management of preterm infants with or without RDS. It is important that infants who have been enrolled in the trials included in this review are followed to assess any effects of inositol supplementation on long-term outcomes in childhood. We do not recommend any additional trials in neonates.
Conflict of interest statement
Dr. Alexandra Howlett has no interests to declare.
Dr. Arne Ohlsson has no interests to declare.
Dr. Nishad Plakkal has no interests to declare.
Figures


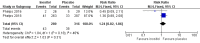
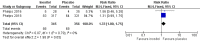












































Update of
-
Inositol in preterm infants at risk for or having respiratory distress syndrome.Cochrane Database Syst Rev. 2015 Feb 4;(2):CD000366. doi: 10.1002/14651858.CD000366.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jul 08;7:CD000366. doi: 10.1002/14651858.CD000366.pub4. PMID: 25927089 Updated.
References
References to studies included in this review
Friedman 1995 {published and unpublished data}
-
- Friedman CA, McVey J, Borne MJ, James M, May WL, Temple DM, et al. Relationship between serum inositol concentration and development of retinopathy of prematurity: A prospective study. Journal of Pediatric Ophthalmology and Strabismus 2000;37(2):79‐86. [PUBMED: 10779265] - PubMed
-
- Friedman CA, Temple DM, Robbins KK, Miller CJ, Rawson JE. Randomized controlled trial of high inositol and calorie supplementation in preterm infants at risk for chronic lung and eye disease. American Academy of Pediatrics Annual Meeting; San Francisco, California, USA. 1995 October.
Hallman 1986 {published data only}
Hallman 1992 {published data only}
-
- Hallman M, Pohjavuori M, Bry K. Inositol supplementation in respiratory distress syndrome. Lung 1990;168 Suppl:877‐82. [PUBMED: 2117207] - PubMed
Phelps 2013 {published data only}
-
- Phelps DL, Ward RM, Williams RL, Watterberg KL, Laptook AR, Wrage LA, et al. on behalf of the Inositol Subcommittee of the Neonatal Research Network. Pharmacokinetics and safety of a single dose of myo‐inositol in preterm infants of 23‐29 wk. E‐PAS2010. 2010:3737.387.
Phelps 2016 {published data only}
Phelps 2018 {published data only}
Additional references
Bell 1978
Bromberger 1986
-
- Bromberger P, Hallman M. Myoinositol in small preterm infants: Relationship between intake and serum concentration. Journal of Pediatric Gastroenterology and Nutrition 1986;5(3):455‐8. [PUBMED: 3088251] - PubMed
Burton 1974
-
- Burton LE, Wells WW. Studies on the development pattern of the enzymes converting glucose‐6‐phosphate to myo‐inositol in the rat. Developmental Biology 1974;37(1):35‐42. [PUBMED: 4362962] - PubMed
Dawson 1961
Eagle 1957
-
- Eagle H, Oyama VI, Levy M, Freeman AE. Myo‐inositol as an essential growth factor for normal and malignant human cells in tissue culture. Journal of Biological Chemistry 1957;266(1):191‐205. [PUBMED: 13428752] - PubMed
Egberts 1986
-
- Egberts J, Noort WA. Gestational age‐dependent changes in plasma inositol levels and surfactant composition in the fetal rat. Pediatric Research 1986;20(1):24‐7. [PUBMED: 3753753] - PubMed
Guadagnino 2012
Guarner 1992
-
- Guarner V, Tordet C, Bourbon JR. Effects of maternal protein‐calorie malnutrition on the phospholipid composition of surfactant isolated from the fetal and neonatal rat lungs. Compensation by inositol and lipid supplementation. Pediatric Research 1992;31(6):629‐35. [DOI: 10.1203/00006450-199206000-00018; PUBMED: 1635827] - DOI - PubMed
Hallman 1980
-
- Hallman M, Epstein BL. Role of myo‐inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Biochemical and Biophysical Research Communications 1980;92(4):1151‐9. [PUBMED: 6245646] - PubMed
Hallman 1984
-
- Hallman M. Effect of intracellular myo‐inositol on the surfactant phospholipid synthesis in the fetal rabbit lung. Biochimica et Biophysica Acta 1984;795(1):67‐78. [PUBMED: 6547857] - PubMed
Hallman 1985
-
- Hallman M, Saugstad OD, Porreco RP, Epstein BL, Gluck L. Role of myo‐inositol in regulation of surfactant phospholipids in the newborn. Early Human Development 1985;10(3‐4):245‐54. [PUBMED: 3838720] - PubMed
Hallman 1987
-
- Hallman M, Arjomaa P, Hoppu K. Inositol supplementation in respiratory distress syndrome: Relationship between serum concentration, renal excretion, and lung effluent phospholipids. Journal of Pediatrics 1987;110(4):604‐10. [PUBMED: 3559811] - PubMed
Hallman 1990
-
- Hallman M, Pohjavuori M, Bry K. Inositol supplementation in respiratory distress syndrome. Lung 1990;168 Suppl:877‐82. [PUBMED: 2117207] - PubMed
Hasan 1974
-
- Hasan SH, Nishigaki I, Tsutsui Y, Yagi K. Studies on myoinositol IX. Morphological examination of the effect of massive doses of myoinositol on the liver and kidney of rat. Journal of Nutritional Science and Vitaminology 1974;20(1):55‐8. [PUBMED: 4836951] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICROP 1984
-
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. The Committee for the Classification of Retinopathy of Prematurity. Archives of Ophthalmology 1984;102(8):1130‐4. [PUBMED: 6547831] - PubMed
Lewin 1978
Manske 2016
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birthweights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] - PubMed
Pereira 1990
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shennan 1988
-
- Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 1988;82(4):527‐32. [PUBMED: 3174313] - PubMed
Soll 1992
-
- Soll RF, McQueen MC. Respiratory distress syndrome. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992.
References to other published versions of this review
Howlett 1997
Howlett 2003
Howlett 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

