HMGB1 and Caveolin-1 related to RPE cell senescence in age-related macular degeneration
- PMID: 31284269
- PMCID: PMC6660032
- DOI: 10.18632/aging.102039
HMGB1 and Caveolin-1 related to RPE cell senescence in age-related macular degeneration
Abstract
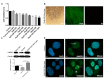
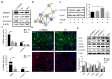
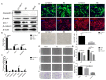
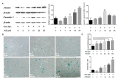
Accumulation of lipofuscin in the retinal pigment epithelium (RPE) is considered a major cause of RPE dysfunction and senescence in age-related macular degeneration (AMD), and N-retinylidene-N-retinylethanolamine (A2E) is the main fluorophore identified in lipofuscin from aged human eyes. Here, human-induced pluripotent stem cell (iPSC)-RPE was generated from healthy individuals to reveal proteomic changes associated with A2E-related RPE cell senescence. A novel RPE cell senescence-related protein, high-mobility group box 1 (HMGB1), was identified based on proteomic mass spectrometry measurements on iPSC-RPE with A2E treatment. Furthermore, HMGB1 upregulated Caveolin-1, which also was related RPE cell senescence. To investigate whether changes in HMGB1 and Caveolin-1 expression under A2E exposure contribute to RPE cell senescence, human ARPE-19 cells were stimulated with A2E; expression of HMGB1, Caveolin-1, tight junction proteins and senescent phenotypes were verified. HMGB1 inhibition alleviated A2E induced cell senescence. Migration of RPE cells was evaluated. Notably, A2E less than or equal to 10μM induced both HMGB1 and Caveolin-1 protein upregulation and HMGB1 translocation, while Caveolin-1 expression was downregulated when there was more than 10μM A2E. Our data indicate that A2E-induced upregulation of HMGB1、Caveolin-1 and HMGB1 release may relate to RPE cell senescence and play a role in the pathogenesis of AMD.
Keywords: A2E; AMD; Caveolin-1; HMGB1; RPE cell senescence.
Conflict of interest statement
Figures


Similar articles
-
Exposure of A2E to blue light promotes ferroptosis in the retinal pigment epithelium.Cell Mol Biol Lett. 2025 Feb 21;30(1):22. doi: 10.1186/s11658-025-00700-2. Cell Mol Biol Lett. 2025. PMID: 39984833 Free PMC article.
-
A novel fluorescence-based assay for measuring A2E removal from human retinal pigment epithelial cells to screen for age-related macular degeneration inhibitors.J Pharm Biomed Anal. 2016 Jan 5;117:560-7. doi: 10.1016/j.jpba.2015.10.010. J Pharm Biomed Anal. 2016. PMID: 26604166
-
Relative Contributions of All-Trans and 11-Cis Retinal to Formation of Lipofuscin and A2E Accumulating in Mouse Retinal Pigment Epithelium.Invest Ophthalmol Vis Sci. 2021 Feb 1;62(2):1. doi: 10.1167/iovs.62.2.1. Invest Ophthalmol Vis Sci. 2021. PMID: 33523199 Free PMC article.
-
A2E and Lipofuscin.Prog Mol Biol Transl Sci. 2015;134:449-63. doi: 10.1016/bs.pmbts.2015.06.005. Epub 2015 Jul 14. Prog Mol Biol Transl Sci. 2015. PMID: 26310170 Review.
-
A2E, a byproduct of the visual cycle.Vision Res. 2003 Dec;43(28):2983-90. doi: 10.1016/s0042-6989(03)00475-9. Vision Res. 2003. PMID: 14611934 Review.
Cited by
-
Pharmacologic activation of autophagy without direct mTOR inhibition as a therapeutic strategy for treating dry macular degeneration.Aging (Albany NY). 2021 Apr 19;13(8):10866-10890. doi: 10.18632/aging.202974. Epub 2021 Apr 19. Aging (Albany NY). 2021. PMID: 33872219 Free PMC article.
-
Lycium barbarum polysaccharide alleviates H2O2-induced premature senescence by downregulating miRNA-34a-5p in ARPE-19 cells.Cell Stress Chaperones. 2025 May;30(3):130-142. doi: 10.1016/j.cstres.2025.03.002. Epub 2025 Mar 18. Cell Stress Chaperones. 2025. PMID: 40112947 Free PMC article.
-
The Impact of Aging on Ocular Diseases: Unveiling Complex Interactions.Aging Dis. 2024 Sep 23;16(5):2803-2830. doi: 10.14336/AD.2024.0850. Aging Dis. 2024. PMID: 39500360 Free PMC article. Review.
-
Senolytic drugs: from discovery to translation.J Intern Med. 2020 Nov;288(5):518-536. doi: 10.1111/joim.13141. Epub 2020 Aug 4. J Intern Med. 2020. PMID: 32686219 Free PMC article. Review.
-
The importance of caveolins and caveolae to dermatology: Lessons from the caves and beyond.Exp Dermatol. 2020 Feb;29(2):136-148. doi: 10.1111/exd.14068. Epub 2020 Jan 10. Exp Dermatol. 2020. PMID: 31845391 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

