Starvation-Induced Differential Virotherapy Using an Oncolytic Measles Vaccine Virus
- PMID: 31284426
- PMCID: PMC6669668
- DOI: 10.3390/v11070614
Starvation-Induced Differential Virotherapy Using an Oncolytic Measles Vaccine Virus
Abstract
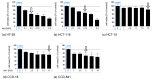
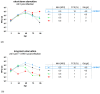
Starvation sensitizes tumor cells to chemotherapy while protecting normal cells at the same time, a phenomenon defined as differential stress resistance. In this study, we analyzed if starvation would also increase the oncolytic potential of an oncolytic measles vaccine virus (MeV-GFP) while protecting normal cells against off-target lysis. Human colorectal carcinoma (CRC) cell lines as well as human normal colon cell lines were subjected to various starvation regimes and infected with MeV-GFP. The applied fasting regimes were either short-term (24 h pre-infection) or long-term (24 h pre- plus 96 h post-infection). Cell-killing features of (i) virotherapy, (ii) starvation, as well as (iii) the combination of both were analyzed by cell viability assays and virus growth curves. Remarkably, while long-term low-serum, standard glucose starvation potentiated the efficacy of MeV-mediated cell killing in CRC cells, it was found to be decreased in normal colon cells. Interestingly, viral replication of MeV-GFP in CRC cells was decreased in long-term-starved cells and increased after short-term low-glucose, low-serum starvation. In conclusion, starvation-based virotherapy has the potential to differentially enhance MeV-mediated oncolysis in the context of CRC cancer patients while protecting normal colon cells from unwanted off-target effects.
Keywords: fasting; measles vaccine virus; oncolysis; starvation; virotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Lee C., Safdie F.M., Raffaghello L., Wei M., Madia F., Parrella E., Hwang D., Cohen P., Bianchi G., Longo V.D. Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res. 2010;70:1564–1572. doi: 10.1158/0008-5472.CAN-09-3228. - DOI - PMC - PubMed
-
- Lee C., Raffaghello L., Brandhorst S., Safdie F.M., Bianchi G., Martin-Montalvo A., Pistoia V., Wei M., Hwang S., Merlino A., et al. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Sci. Transl. Med. 2012;4:124ra27. doi: 10.1126/scitranslmed.3003293. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

