The Clinical Perspective on Hepatitis E
- PMID: 31284447
- PMCID: PMC6669652
- DOI: 10.3390/v11070617
The Clinical Perspective on Hepatitis E
Abstract
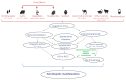
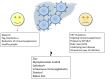
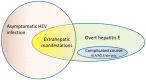
Every year, there are an estimated 20 million hepatitis E virus (HEV) infections worldwide, leading to an estimated 3.3 million symptomatic cases of hepatitis E. HEV is largely circulating in the west and is associated with several hepatic and extrahepatic diseases. HEV Genotype 1 and 2 infections are waterborne and causative for epidemics in the tropics, while genotype 3 and 4 infections are zoonotic diseases and are mainly transmitted by ingestion of undercooked pork in industrialized nations. The clinical course of these infections differs: genotype 1 and 2 infection can cause acute illness and can lead to acute liver failure (ALF) or acute on chronic liver failure (ACLF) with a high mortality rate of 20% in pregnant women. In contrast, the majority of HEV GT-3 and -4 infections have a clinically asymptomatic course and only rarely lead to acute on chronic liver failure in elderly or patients with underlying liver disease. Immunosuppressed individuals infected with genotype 3 or 4 may develop chronic hepatitis E, which then can lead to life-threatening cirrhosis. Furthermore, several extra-hepatic manifestations affecting various organs have been associated with ongoing or previous HEV infections but the causal link for many of them still needs to be proven. There is no approved specific therapy for the treatment of acute or chronic HEV GT-3 or -4 infections but off-label use of ribavirin has been demonstrated to be safe and effective in the majority of patients. However, in approximately 15% of chronically HEV infected patients, cure is not possible.
Keywords: HEV; Hepatitis E.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Adlhoch C., Avellon A., Baylis S.A., Ciccaglione A.R., Couturier E., de Sousa R., Epstein J., Ethelberg S., Faber M., Feher A., et al. Hepatitis e virus: Assessment of the epidemiological situation in humans in europe, 2014/15. J. Clin. Virol. 2016;82:9–16. doi: 10.1016/j.jcv.2016.06.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

