Glucagon Receptor Signaling and Glucagon Resistance
- PMID: 31284506
- PMCID: PMC6651628
- DOI: 10.3390/ijms20133314
Glucagon Receptor Signaling and Glucagon Resistance
Abstract
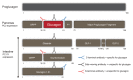
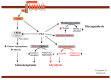
Hundred years after the discovery of glucagon, its biology remains enigmatic. Accurate measurement of glucagon has been essential for uncovering its pathological hypersecretion that underlies various metabolic diseases including not only diabetes and liver diseases but also cancers (glucagonomas). The suggested key role of glucagon in the development of diabetes has been termed the bihormonal hypothesis. However, studying tissue-specific knockout of the glucagon receptor has revealed that the physiological role of glucagon may extend beyond blood-glucose regulation. Decades ago, animal and human studies reported an important role of glucagon in amino acid metabolism through ureagenesis. Using modern technologies such as metabolomic profiling, knowledge about the effects of glucagon on amino acid metabolism has been expanded and the mechanisms involved further delineated. Glucagon receptor antagonists have indirectly put focus on glucagon's potential role in lipid metabolism, as individuals treated with these antagonists showed dyslipidemia and increased hepatic fat. One emerging field in glucagon biology now seems to include the concept of hepatic glucagon resistance. Here, we discuss the roles of glucagon in glucose homeostasis, amino acid metabolism, and lipid metabolism and present speculations on the molecular pathways causing and associating with postulated hepatic glucagon resistance.
Keywords: alpha cell; amino acids; diabetes; glucose; hyperaminoacidemia; hyperglucagonemia; liver–alpha cell axis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Kimball C.P., Murlin J.R. Aqueous Extracts of Pancreas: III. Some Precipitation Reactions of Insulin. J. Biol. Chem. 1923;58:337–346.
-
- Sutherland E.W., Cori C.F., Haynes R., Olsen N.S. Purification of the hyperglycemic-glycogenolytic factor from insulin and from gastric mucosa. J. Biol. Chem. 1949;180:825–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

