Steroids as Environmental Compounds Recalcitrant to Degradation: Genetic Mechanisms of Bacterial Biodegradation Pathways
- PMID: 31284586
- PMCID: PMC6678751
- DOI: 10.3390/genes10070512
Steroids as Environmental Compounds Recalcitrant to Degradation: Genetic Mechanisms of Bacterial Biodegradation Pathways
Abstract
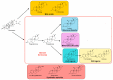
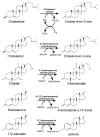
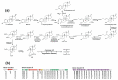
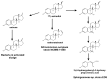
Steroids are perhydro-1,2-cyclopentanophenanthrene derivatives that are almost exclusively synthesised by eukaryotic organisms. Since the start of the Anthropocene, the presence of these molecules, as well as related synthetic compounds (ethinylestradiol, dexamethasone, and others), has increased in different habitats due to farm and municipal effluents and discharge from the pharmaceutical industry. In addition, the highly hydrophobic nature of these molecules, as well as the absence of functional groups, makes them highly resistant to biodegradation. However, some environmental bacteria are able to modify or mineralise these compounds. Although steroid-metabolising bacteria have been isolated since the beginning of the 20th century, the genetics and catabolic pathways used have only been characterised in model organisms in the last few decades. Here, the metabolic alternatives used by different bacteria to metabolise steroids (e.g., cholesterol, bile acids, testosterone, and other steroid hormones), as well as the organisation and conservation of the genes involved, are reviewed.
Keywords: 2,3-seco pathway; 4,5-seco pathway; 9,10-seco pathway; bile acids; biodegradation; steroid hormones; sterols.
Conflict of interest statement
The authors declare no conflict of interest. Those funding our research had no role in the design of the study, in the collection, analyses, or interpretation of data, or in the writing of the manuscript.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

