Neutrophil elastase promotes Leishmania donovani infection via interferon-β
- PMID: 31284755
- PMCID: PMC6766642
- DOI: 10.1096/fj.201900524R
Neutrophil elastase promotes Leishmania donovani infection via interferon-β
Abstract
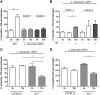
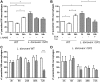
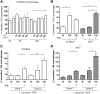
Visceral leishmaniasis is a deadly illness caused by Leishmania donovani that provokes liver and spleen inflammation and tissue destruction. In cutaneous leishmaniasis, the protein of L. major, named inhibitor of serine peptidases (ISP) 2, inactivates neutrophil elastase (NE) present at the macrophage surface, resulting in blockade of TLR4 activation, prevention of TNF-α and IFN-β production, and parasite survival. We report poor intracellular growth of L. donovani in macrophages from knockout mice for NE (ela-/-), TLR4, or TLR2. NE and TLR4 colocalized with the parasite in the parasitophorous vacuole. Parasite load in the liver and spleen of ela-/- mice were reduced and accompanied by increased NO and decreased TGF-β production. Expression of ISP2 was not detected in L. donovani, and a transgenic line constitutively expressing ISP2, displayed poor intracellular growth in macrophages and decreased burden in mice. Infected ela-/- macrophages displayed significantly lower IFN-β mRNA than background mice macrophages, and the intracellular growth was fully restored by exogenous IFN-β. We propose that L. donovani utilizes the host NE-TLR machinery to induce IFN-β necessary for parasite survival and growth during early infection. Low or absent expression of parasite ISP2 in L. donovani is necessary to preserve the activation of the NE-TLR pathway.-Dias, B. T., Dias-Teixeira, K. L., Godinho, J. P., Faria, M. S., Calegari-Silva, T., Mukhtar, M. M., Lopes, U. G., Mottram, J. C., Lima, A. P. C. A. Neutrophil elastase promotes Leishmania donovani infection via interferon-β.
Keywords: ISP; inhibitor; serine protease; toll.
Conflict of interest statement
The authors thank Leticia Maneiras (Instituto de Biofisica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro) for technical assistance, Dr. Bernhard Ryffel [Centre de Recherche Scientifique, (CNRS), Orleans, France] for the donation of
Figures







References
-
- WHO Available at: http://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed March 14, 2019
-
- Kaye P. M., Aebischer T. (2011) Visceral leishmaniasis: immunology and prospects for a vaccine. Clin. Microbiol. Infect. 17, 1462–1470 - PubMed
-
- Peacock C. S., Seeger K., Harris D., Murphy L., Ruiz J. C., Quail M. A., Peters N., Adlem E., Tivey A., Aslett M., Kerhornou A., Ivens A., Fraser A., Rajandream M. A., Carver T., Norbertczak H., Chillingworth T., Hance Z., Jagels K., Moule S., Ormond D., Rutter S., Squares R., Whitehead S., Rabbinowitsch E., Arrowsmith C., White B., Thurston S., Bringaud F., Baldauf S. L., Faulconbridge A., Jeffares D., Depledge D. P., Oyola S. O., Hilley J. D., Brito L. O., Tosi L. R., Barrell B., Cruz A. K., Mottram J. C., Smith D. F., Berriman M. (2007) Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 39, 839–847 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

