Asparaginase immune complexes induce Fc-γRIII-dependent hypersensitivity in naive mice
- PMID: 31284767
- PMCID: PMC6766650
- DOI: 10.1096/fj.201900857
Asparaginase immune complexes induce Fc-γRIII-dependent hypersensitivity in naive mice
Abstract
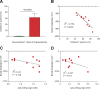
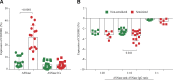
Asparaginase (ASNase) is an important drug for the treatment of leukemias. However, hypersensitivity to ASNase can increase the risk of leukemia relapse. Two mechanisms of ASNase hypersensitivity have been identified in mice. The existence of a pathway involving anti-ASNase IgG and Fc-γ receptor III (Fc-γRIII) implies that IgG and ASNase immune complexes (ICs) could directly induce hypersensitivity. The aim of this study was to detect ASNase ICs in mice after hypersensitivity reactions and determine their role in hypersensitivity. Protein G beads were used to detect plasma ASNase ICs by flow cytometry. Anti-ASNase IgG was purified from the plasma of sensitized mice, and ASNase ICs were prepared ex vivo at various ratios of ASNase to anti-ASNase IgG. The levels of ASNase ICs detected after hypersensitivity reactions correlated with reaction severity (R2 = 0.796; P = 0.0005). ASNase ICs prepared ex vivo required high levels of anti-ASNase IgG for formation, and binding to naive and sensitized immune cells depended on soluble anti-ASNase IgG, antigen:antibody ratio, and Fc-γRIII. Similarly, basophil activation by ASNase ICs depended on the antigen:antibody ratio and Fc-γRIII. Consistent with the ex vivo results, naive mice receiving ASNase ICs developed hypersensitivity reactions. Our data demonstrate that ASNase ICs can directly contribute to the onset and severity of ASNase hypersensitivity.-Rathod, S., Ramsey, M., DiGiorgio, D., Berrios, R., Finkelman, F. D., Fernandez, C. A. Asparaginase immune complexes induce Fc-γRIII-dependent hypersensitivity in naive mice.
Keywords: anaphylaxis; antidrug antibodies; basophil activation test; immunogenicity; leukemia.
Conflict of interest statement
This study was supported by the University of Pittsburgh School of Pharmacy (Pittsburgh, PA, USA) and U.S. National Institutes of Health, National Cancer Institute Grant RO1 CA216815. The authors declare no conflicts of interest.
Figures



References
-
- Müller H. J., Boos J. (1998) Use of L-asparaginase in childhood ALL. Crit. Rev. Oncol. Hematol. 28, 97–113 - PubMed
-
- Avramis V. I., Sencer S., Periclou A. P., Sather H., Bostrom B. C., Cohen L. J., Ettinger A. G., Ettinger L. J., Franklin J., Gaynon P. S. J. B., Hilden J. M., Lange B., Majlessipour F., Mathew P., Needle M., Neglia J., Reaman G., Holcenberg J. S., Stork L. (2002) A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood 99, 1986–1994 - PubMed
-
- Liu C., Kawedia J. D., Cheng C., Pei D., Fernandez C. A., Cai X., Crews K. R., Kaste S. C., Panetta J. C., Bowman W. P. J. L., Jeha S., Sandlund J. T., Evans W. E., Pui C. H., Relling M. V. (2012) Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia 26, 2303–2309 - PMC - PubMed
-
- Oettgen H. F., Stephenson P. A., Schwartz M. K., Leeper R. D., Tallal L., Tan C. C., Clarkson B. D., Golbey R. B., Krakoff I. H., Karnofsky D. A., Murphy M. L., Burchenal J. H. (1970) Toxicity of E. coli L-asparaginase in man. Cancer 25, 253–278 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

