Analysis of novel hyperosmotic shock response suggests 'beads in liquid' cytosol structure
- PMID: 31285266
- PMCID: PMC6679407
- DOI: 10.1242/bio.044529
Analysis of novel hyperosmotic shock response suggests 'beads in liquid' cytosol structure
Abstract
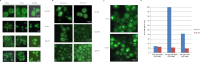
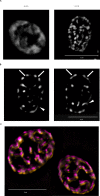
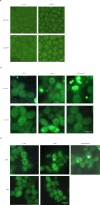
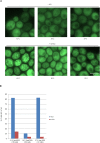
Proteins can aggregate in response to stresses, including hyperosmotic shock. Formation and disassembly of aggregates is a relatively slow process. We describe a novel instant response of the cell to hyperosmosis, during which chaperones and other proteins form numerous foci with properties uncharacteristic of classical aggregates. These foci appeared/disappeared seconds after shock onset/removal, in close correlation with cell volume changes. Genome-wide and targeted testing revealed chaperones, metabolic enzymes, P-body components and amyloidogenic proteins in the foci. Most of these proteins can form large assemblies and for some, the assembled state was pre-requisite for participation in foci. A genome-wide screen failed to identify genes whose absence prevented foci participation by Hsp70. Shapes of and interconnections between foci, revealed by super-resolution microscopy, indicated that the foci were compressed between other entities. Based on our findings, we suggest a new model of cytosol architecture as a collection of numerous gel-like regions suspended in a liquid network. This network is reduced in volume in response to hyperosmosis and forms small pockets between the gel-like regions.
Keywords: Aggregation; Amyloid; Chaperone; Cytoplasm; Foci; Hyperosmotic shock; Liquid–liquid phase separation; P-bodies; Yeast.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

