Reduced mTORC1-signalling in retinal progenitor cells leads to visual pathway dysfunction
- PMID: 31285269
- PMCID: PMC6737973
- DOI: 10.1242/bio.044370
Reduced mTORC1-signalling in retinal progenitor cells leads to visual pathway dysfunction
Abstract
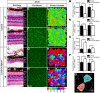
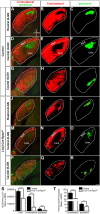
Development of the vertebrate central nervous system involves the co-ordinated differentiation of progenitor cells and the establishment of functional neural networks. This neurogenic process is driven by both intracellular and extracellular cues that converge on the mammalian target of rapamycin complex 1 (mTORC1). Here we demonstrate that mTORC1-signalling mediates multi-faceted roles during central nervous system development using the mouse retina as a model system. Downregulation of mTORC1-signalling in retinal progenitor cells by conditional ablation of Rptor leads to proliferation deficits and an over-production of retinal ganglion cells during embryonic development. In contrast, reduced mTORC1-signalling in postnatal animals leads to temporal deviations in programmed cell death and the consequent production of asymmetric retinal ganglion cell mosaics and associated loss of axonal termination topographies in the dorsal lateral geniculate nucleus of adult mice. In combination these developmental defects induce visually mediated behavioural deficits. These collective observations demonstrate that mTORC1-signalling mediates critical roles during visual pathway development and function.
Keywords: RGCs; Raptor; Retina; Visual cliff test; dLGN; mTORC1.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
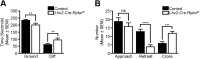
Similar articles
-
Reduced mTORC1-signaling in progenitor cells leads to retinal lamination deficits.Dev Dyn. 2024 Oct;253(10):922-939. doi: 10.1002/dvdy.707. Epub 2024 Mar 28. Dev Dyn. 2024. PMID: 38546215
-
A novel mouse model of tuberous sclerosis complex (TSC): eye-specific Tsc1-ablation disrupts visual-pathway development.Dis Model Mech. 2015 Dec;8(12):1517-29. doi: 10.1242/dmm.021972. Epub 2015 Oct 8. Dis Model Mech. 2015. PMID: 26449264 Free PMC article.
-
Reduced mTORC1-signaling in retinal ganglion cells leads to vascular retinopathy.Dev Dyn. 2022 Feb;251(2):321-335. doi: 10.1002/dvdy.389. Epub 2021 Jun 25. Dev Dyn. 2022. PMID: 34148274
-
Development of On and Off retinal pathways and retinogeniculate projections.Prog Retin Eye Res. 2004 Jan;23(1):31-51. doi: 10.1016/j.preteyeres.2003.10.001. Prog Retin Eye Res. 2004. PMID: 14766316 Review.
-
'Hidden lamination' in the dorsal lateral geniculate nucleus: the functional organization of this thalamic region in the rat.Brain Res. 1988 Apr-Jun;472(2):119-37. doi: 10.1016/0165-0173(88)90017-3. Brain Res. 1988. PMID: 3289687 Review.
Cited by
-
Crosstalk between the mTOR pathway and primary cilia in human diseases.Curr Top Dev Biol. 2023;155:1-37. doi: 10.1016/bs.ctdb.2023.09.004. Epub 2023 Nov 4. Curr Top Dev Biol. 2023. PMID: 38043949 Free PMC article. Review.
-
mTORC1 regulates high levels of protein synthesis in retinal ganglion cells of adult mice.J Biol Chem. 2022 Jun;298(6):101944. doi: 10.1016/j.jbc.2022.101944. Epub 2022 Apr 18. J Biol Chem. 2022. PMID: 35447116 Free PMC article.
-
Role of the Internal Limiting Membrane in Structural Engraftment and Topographic Spacing of Transplanted Human Stem Cell-Derived Retinal Ganglion Cells.Stem Cell Reports. 2021 Jan 12;16(1):149-167. doi: 10.1016/j.stemcr.2020.12.001. Epub 2020 Dec 30. Stem Cell Reports. 2021. PMID: 33382979 Free PMC article.
-
Role of mTORC1 activity during early retinal development and lamination in human-induced pluripotent stem cell-derived retinal organoids.Cell Death Discov. 2022 Feb 8;8(1):56. doi: 10.1038/s41420-022-00837-5. Cell Death Discov. 2022. PMID: 35136019 Free PMC article.
-
Beyond Genetics: The Role of Metabolism in Photoreceptor Survival, Development and Repair.Front Cell Dev Biol. 2022 May 18;10:887764. doi: 10.3389/fcell.2022.887764. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35663397 Free PMC article. Review.
References
-
- Bansal A., Singer J. H., Hwang B. J., Xu W., Beaudet A. and Feller M. B. (2000). Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J. Neurosci. 20, 7672-7681. 10.1523/JNEUROSCI.20-20-07672.2000 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

