Trpc5 deficiency causes hypoprolactinemia and altered function of oscillatory dopamine neurons in the arcuate nucleus
- PMID: 31285329
- PMCID: PMC6660783
- DOI: 10.1073/pnas.1905705116
Trpc5 deficiency causes hypoprolactinemia and altered function of oscillatory dopamine neurons in the arcuate nucleus
Abstract
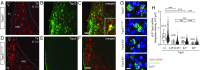
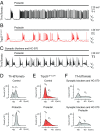
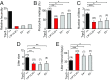
Dopamine neurons of the hypothalamic arcuate nucleus (ARC) tonically inhibit the release of the protein hormone prolactin from lactotropic cells in the anterior pituitary gland and thus play a central role in prolactin homeostasis of the body. Prolactin, in turn, orchestrates numerous important biological functions such as maternal behavior, reproduction, and sexual arousal. Here, we identify the canonical transient receptor potential channel Trpc5 as an essential requirement for normal function of dopamine ARC neurons and prolactin homeostasis. By analyzing female mice carrying targeted mutations in the Trpc5 gene including a conditional Trpc5 deletion, we show that Trpc5 is required for maintaining highly stereotyped infraslow membrane potential oscillations of dopamine ARC neurons. Trpc5 is also required for eliciting prolactin-evoked tonic plateau potentials in these neurons that are part of a regulatory feedback circuit. Trpc5 mutant females show severe prolactin deficiency or hypoprolactinemia that is associated with irregular reproductive cyclicity, gonadotropin imbalance, and impaired reproductive capabilities. These results reveal a previously unknown role for the cation channel Trpc5 in prolactin homeostasis of female mice and provide strategies to explore the genetic basis of reproductive disorders and other malfunctions associated with defective prolactin regulation in humans.
Keywords: HC-070; Trpc5 channelopathy; dopamine; hypothalamus; prolactin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Clapham D. E., Montell C., Schultz G., Julius D.; International Union of Pharmacology , International union of pharmacology. XLIII. Compendium of voltage-gated ion channels: Transient receptor potential channels. Pharmacol. Rev. 55, 591–596 (2003). - PubMed
-
- Zholos A., “TRPC5” in Mammalian Transient Receptor Potential (TRP) Cation Channels, Nilius B., Flockerzi V., Eds. (Springer, Berlin, Heidelberg, 2014), vol. 1, pp. 129–156.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

