Global-level population genomics reveals differential effects of geography and phylogeny on horizontal gene transfer in soil bacteria
- PMID: 31285337
- PMCID: PMC6660780
- DOI: 10.1073/pnas.1900056116
Global-level population genomics reveals differential effects of geography and phylogeny on horizontal gene transfer in soil bacteria
Abstract
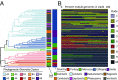
Although microorganisms are known to dominate Earth's biospheres and drive biogeochemical cycling, little is known about the geographic distributions of microbial populations or the environmental factors that pattern those distributions. We used a global-level hierarchical sampling scheme to comprehensively characterize the evolutionary relationships and distributional limitations of the nitrogen-fixing bacterial symbionts of the crop chickpea, generating 1,027 draft whole-genome sequences at the level of bacterial populations, including 14 high-quality PacBio genomes from a phylogenetically representative subset. We find that diverse Mesorhizobium taxa perform symbiosis with chickpea and have largely overlapping global distributions. However, sampled locations cluster based on the phylogenetic diversity of Mesorhizobium populations, and diversity clusters correspond to edaphic and environmental factors, primarily soil type and latitude. Despite long-standing evolutionary divergence and geographic isolation, the diverse taxa observed to nodulate chickpea share a set of integrative conjugative elements (ICEs) that encode the major functions of the symbiosis. This symbiosis ICE takes 2 forms in the bacterial chromosome-tripartite and monopartite-with tripartite ICEs confined to a broadly distributed superspecies clade. The pairwise evolutionary relatedness of these elements is controlled as much by geographic distance as by the evolutionary relatedness of the background genome. In contrast, diversity in the broader gene content of Mesorhizobium genomes follows a tight linear relationship with core genome phylogenetic distance, with little detectable effect of geography. These results illustrate how geography and demography can operate differentially on the evolution of bacterial genomes and offer useful insights for the development of improved technologies for sustainable agriculture.
Keywords: integrative conjugative element; microbial ecology; nitrogen fixation; population genomics; symbiosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pace N. R., A molecular view of microbial diversity and the biosphere. Science 276, 734–740 (1997). - PubMed

