Differences in placental capillary shear stress in fetal growth restriction may affect endothelial cell function and vascular network formation
- PMID: 31285454
- PMCID: PMC6614400
- DOI: 10.1038/s41598-019-46151-6
Differences in placental capillary shear stress in fetal growth restriction may affect endothelial cell function and vascular network formation
Abstract
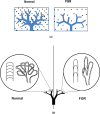
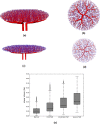
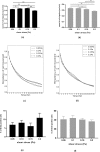
Fetal growth restriction (FGR) affects 5-10% of pregnancies, leading to clinically significant fetal morbidity and mortality. FGR placentae frequently exhibit poor vascular branching, but the mechanisms driving this are poorly understood. We hypothesize that vascular structural malformation at the organ level alters microvascular shear stress, impairing angiogenesis. A computational model of placental vasculature predicted elevated placental micro-vascular shear stress in FGR placentae (0.2 Pa in severe FGR vs 0.05 Pa in normal placentae). Endothelial cells cultured under predicted FGR shear stresses migrated significantly slower and with greater persistence than in shear stresses predicted in normal placentae. These cell behaviors suggest a dominance of vessel elongation over branching. Taken together, these results suggest (1) poor vascular development increases vessel shear stress, (2) increased shear stress induces cell behaviors that impair capillary branching angiogenesis, and (3) impaired branching angiogenesis continues to drive elevated shear stress, jeopardizing further vascular formation. Inadequate vascular branching early in gestation could kick off this cyclic loop and continue to negatively impact placental angiogenesis throughout gestation.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Mesenchymal Stem/Stromal Cells from the Placentae of Growth Restricted Pregnancies Are Poor Stimulators of Angiogenesis.Stem Cell Rev Rep. 2020 Jun;16(3):557-568. doi: 10.1007/s12015-020-09959-8. Stem Cell Rev Rep. 2020. PMID: 32080795
-
Fetoplacental vascular alterations associated with fetal growth restriction.Placenta. 2014 Oct;35(10):808-15. doi: 10.1016/j.placenta.2014.07.013. Epub 2014 Aug 6. Placenta. 2014. PMID: 25145956
-
Effect of Bushen Yiqi Huoxue recipe on placental vasculature in pregnant rats with fetal growth restriction induced by passive smoking.J Huazhong Univ Sci Technolog Med Sci. 2013 Apr;33(2):293-302. doi: 10.1007/s11596-013-1114-y. Epub 2013 Apr 17. J Huazhong Univ Sci Technolog Med Sci. 2013. PMID: 23592147
-
Regulation of vascular growth and function in the human placenta.Reproduction. 2009 Dec;138(6):895-902. doi: 10.1530/REP-09-0092. Epub 2009 May 26. Reproduction. 2009. PMID: 19470597 Review.
-
The placenta in fetal growth restriction: What is going wrong?Placenta. 2020 Jul;96:10-18. doi: 10.1016/j.placenta.2020.05.003. Epub 2020 May 11. Placenta. 2020. PMID: 32421528 Review.
Cited by
-
Angiogenesis in Tissue Engineering: As Nature Intended?Front Bioeng Biotechnol. 2020 Mar 20;8:188. doi: 10.3389/fbioe.2020.00188. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32266227 Free PMC article. Review.
-
Bioengineering Approaches for Placental Research.Ann Biomed Eng. 2021 Aug;49(8):1805-1818. doi: 10.1007/s10439-020-02714-7. Epub 2021 Jan 8. Ann Biomed Eng. 2021. PMID: 33420547 Review.
-
Analysis of Placental Arteriovenous Formation Reveals New Insights Into Embryos With Congenital Heart Defects.Front Genet. 2022 Jan 19;12:806136. doi: 10.3389/fgene.2021.806136. eCollection 2021. Front Genet. 2022. PMID: 35126469 Free PMC article.
-
Ultrasound Microvessel Imaging of the Human Placenta Demonstrates Altered Vessel Densities in Fetal Growth Restriction With Vascular and Immune Pathologies: A Pilot Case-Control Study.J Ultrasound Med. 2025 Feb;44(2):285-299. doi: 10.1002/jum.16604. Epub 2024 Oct 18. J Ultrasound Med. 2025. PMID: 39422170 Free PMC article.
-
Multi-scale Modelling of Shear Stress on the Syncytiotrophoblast: Could Maternal Blood Flow Impact Placental Function Across Gestation?Ann Biomed Eng. 2023 Jun;51(6):1256-1269. doi: 10.1007/s10439-022-03129-2. Epub 2023 Feb 6. Ann Biomed Eng. 2023. PMID: 36745293 Free PMC article.
References
-
- Sadler, T. W. & Langmans, J. Langman’s medical embryology. (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, ©2012 2012).
-
- Wang, Y. & Zhao, S. Vascular Biology of the Placenta. (2010 by Morgan & Claypool Life Sciences. 2010). - PubMed
-
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

