Phosphorylation of p23-1 cochaperone by protein kinase CK2 affects root development in Arabidopsis
- PMID: 31285503
- PMCID: PMC6614504
- DOI: 10.1038/s41598-019-46327-0
Phosphorylation of p23-1 cochaperone by protein kinase CK2 affects root development in Arabidopsis
Abstract
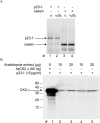
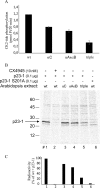
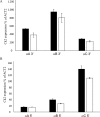
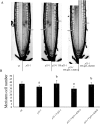
Root growth is a fundamental process in plants and assures nutrient and water uptake required for efficient photosynthesis and metabolism. Postembryonic development of roots is controlled by the functionality of the meristem. Several hormones and signaling molecules regulate the size of the meristem, and among them, auxins play a major role. Protein kinase CK2, along with the chaperone protein HSP90, has been found to be involved in the regulation of auxin transport. Here, we show that p23-1, a cochaperone of HSP90, is phosphorylated by CK2 in Arabidopsis. We identified Ser201 as the major CK2 target site in p23-1 and demonstrated that phosphorylation of this site is necessary for normal root development. Moreover, we shed light on the nature of CK2 in Arabidopsis, showing that the three catalytic isoforms, CK2 αA, αB and αC, are proteins of approximately 40 kDa. Our results increase knowledge of the connection among HSP90, p23-1 and CK2 in Arabidopsis, suggesting the existence of a possible common root development mechanism controlled by these signaling molecules.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The co-chaperone p23 controls root development through the modulation of auxin distribution in the Arabidopsis root meristem.J Exp Bot. 2015 Aug;66(16):5113-22. doi: 10.1093/jxb/erv330. Epub 2015 Jul 10. J Exp Bot. 2015. PMID: 26163704 Free PMC article.
-
The p23 co-chaperone protein is a novel substrate of CK2 in Arabidopsis.Mol Cell Biochem. 2011 Oct;356(1-2):245-54. doi: 10.1007/s11010-011-0969-0. Epub 2011 Jul 7. Mol Cell Biochem. 2011. PMID: 21735091
-
Functional interplay between protein kinase CK2 and salicylic acid sustains PIN transcriptional expression and root development.Plant J. 2014 May;78(3):411-23. doi: 10.1111/tpj.12481. Epub 2014 Apr 2. Plant J. 2014. PMID: 24547808
-
Deciphering Auxin-Ethylene Crosstalk at a Systems Level.Int J Mol Sci. 2018 Dec 14;19(12):4060. doi: 10.3390/ijms19124060. Int J Mol Sci. 2018. PMID: 30558241 Free PMC article. Review.
-
Proteomics in deciphering the auxin commitment in the Arabidopsis thaliana root growth.J Proteome Res. 2013 Nov 1;12(11):4685-701. doi: 10.1021/pr400697s. Epub 2013 Oct 1. J Proteome Res. 2013. PMID: 24032454 Review.
Cited by
-
Hypoviral-regulated HSP90 co-chaperone p23 (CpCop23) determines the colony morphology, virulence, and viral response of chestnut blight fungus Cryphonectria parasitica.Mol Plant Pathol. 2023 May;24(5):413-424. doi: 10.1111/mpp.13308. Epub 2023 Feb 10. Mol Plant Pathol. 2023. PMID: 36762926 Free PMC article.
-
Identification of Candidate Genes for Root Traits Using Genotype-Phenotype Association Analysis of Near-Isogenic Lines in Hexaploid Wheat (Triticum aestivum L.).Int J Mol Sci. 2021 Mar 30;22(7):3579. doi: 10.3390/ijms22073579. Int J Mol Sci. 2021. PMID: 33808237 Free PMC article.
-
CK2 promotes jasmonic acid signaling response by phosphorylating MYC2 in Arabidopsis.Nucleic Acids Res. 2023 Jan 25;51(2):619-630. doi: 10.1093/nar/gkac1213. Nucleic Acids Res. 2023. PMID: 36546827 Free PMC article.
-
Phosphoproteomic insights into the regulation of root length in rice (Oryza sativa L. cv. KDML 105): uncovering key events and pathways involving phosphorylated proteins.PeerJ. 2025 Jul 4;13:e19361. doi: 10.7717/peerj.19361. eCollection 2025. PeerJ. 2025. PMID: 40625925 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

