A Rationally Designed Humanized Antibody Selective for Amyloid Beta Oligomers in Alzheimer's Disease
- PMID: 31285517
- PMCID: PMC6614461
- DOI: 10.1038/s41598-019-46306-5
A Rationally Designed Humanized Antibody Selective for Amyloid Beta Oligomers in Alzheimer's Disease
Abstract
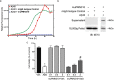
Advances in the understanding of Alzheimer's disease (AD) suggest that pathogenesis is not directly related to plaque burden, but rather to soluble toxic amyloid-beta oligomers (AßO). Therapeutic antibodies targeting Aß monomers and/or plaque have shown limited efficacy and dose-limiting adverse events in clinical trials. These findings suggest that antibodies capable of selectively neutralizing toxic AßO may achieve improved efficacy and safety. To this end, we generated monoclonal antibodies against a conformational Aß epitope predicted by computational modeling to be presented on toxic AßO but not monomers or fibrils. The resulting lead antibody, PMN310, showed the desired AßO-selective binding profile. In vitro, PMN310 inhibited AßO propagation and toxicity. In vivo, PMN310 prevented AßO-induced loss of memory formation and reduced synaptic loss and inflammation. A humanized version (huPMN310) compared favorably to other Aß-directed antibodies showing a lack of adverse event-associated binding to Aß deposits in AD brains, and greater selective binding to AßO-enriched AD brain fractions that contain synaptotoxic Aß species. Systemic administration of huPMN310 in mice resulted in brain exposure and kinetics comparable to those of other therapeutic human monoclonal antibodies. Greater selectivity for AßO and the potential to safely administer high doses of huPMN310 are expected to result in enhanced safety and therapeutic potency.
Conflict of interest statement
S.S.P., N.R.C. and J.M.K. are Chief Physics Officer, Chief Scientific Officer and Chief Development Officer of ProMIS Neurosciences, respectively. S.S.P. is the inventor on patent application PCT/CA2016/051306 for Collective Coordinates computational modeling (applicant being University of British Columbia). S.S.P., N.R.C.; S.S.P., N.R.C. are inventors on patent application PCT/CA2016/051303 (applicant being University of British Columbia) which has entered the national phase in US, CA, EP, CN, JP, AU, IN and SK, and N.R.C., S.S.P., J.M.K., E.G. and J.M.S. are inventors on patent applications US. Serial No. 16/148,601 and PCT/CA2017/050866, which has entered national phase in in US, CA, EP, CN, JP, AU, IN and SK (co-applicants for both being University of British Columbia and ProMIS Neurosciences). The patent applications describe immunogens, antibodies and methods of their making as well as their use. Patent applications owned by the University of British Columbia are licensed to ProMIS Neurosciences. The work presented was financially supported by ProMIS. E.G., J.M.S., X.P., S.S.P., J.M.K. and N.R.C. have received consultation compensation from ProMIS. E.G., J.M.S. and S.S.P. possess ProMIS stock options. N.R.C. and J.M.K. possess ProMIS shares and stock options. B.Z., J.W., C.W. and I.M. declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

