Cryo-EM structures reveal coordinated domain motions that govern DNA cleavage by Cas9
- PMID: 31285607
- PMCID: PMC6842131
- DOI: 10.1038/s41594-019-0258-2
Cryo-EM structures reveal coordinated domain motions that govern DNA cleavage by Cas9
Abstract
The RNA-guided Cas9 endonuclease from Streptococcus pyogenes is a single-turnover enzyme that displays a stable product state after double-stranded-DNA cleavage. Here, we present cryo-EM structures of precatalytic, postcatalytic and product states of the active Cas9-sgRNA-DNA complex in the presence of Mg2+. In the precatalytic state, Cas9 adopts the 'checkpoint' conformation with the HNH nuclease domain positioned far away from the DNA. Transition to the postcatalytic state involves a dramatic ~34-Å swing of the HNH domain and disorder of the REC2 recognition domain. The postcatalytic state captures the cleaved substrate bound to the catalytically competent HNH active site. In the product state, the HNH domain is disordered, REC2 returns to the precatalytic conformation, and additional interactions of REC3 and RuvC with nucleic acids are formed. The coupled domain motions and interactions between the enzyme and the RNA-DNA hybrid provide new insights into the mechanism of genome editing by Cas9.
Figures

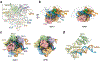

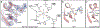

References
Methods-only References:
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

