Regulatory T-cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner
- PMID: 31285764
- PMCID: PMC6599663
- DOI: 10.7150/thno.32734
Regulatory T-cells regulate neonatal heart regeneration by potentiating cardiomyocyte proliferation in a paracrine manner
Abstract
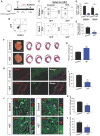
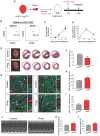
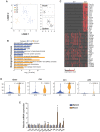
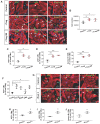
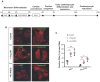
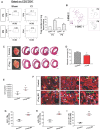
The neonatal mouse heart is capable of transiently regenerating after injury from postnatal day (P) 0-7 and macrophages are found important in this process. However, whether macrophages alone are sufficient to orchestrate this regeneration; what regulates cardiomyocyte proliferation; why cardiomyocytes do not proliferate after P7; and whether adaptive immune cells such as regulatory T-cells (Treg) influence neonatal heart regeneration have less studied. Methods: We employed both loss- and gain-of-function transgenic mouse models to study the role of Treg in neonatal heart regeneration. In loss-of-function studies, we treated mice with the lytic anti-CD25 antibody that specifically depletes Treg; or we treated FOXP3DTR with diphtheria toxin that specifically ablates Treg. In gain-of-function studies, we adoptively transferred hCD2+ Treg from NOD.Foxp3hCD2 to NOD/SCID that contain Treg as the only T-cell population. Furthermore, we performed single-cell RNA-sequencing of Treg to uncover paracrine factors essential for cardiomyocyte proliferation. Results: Unlike their wild type counterparts, NOD/SCID mice that are deficient in T-cells but harbor macrophages fail to regenerate their injured myocardium at as early as P3. During the first week of injury, Treg are recruited to the injured cardiac muscle but their depletion contributes to more severe cardiac fibrosis. On the other hand, adoptive transfer of Treg results in mitigated fibrosis and enhanced proliferation and function of the injured cardiac muscle. Mechanistically, single-cell transcriptomic profiling reveals that Treg could be a source of regenerative factors. Treg directly promote proliferation of both mouse and human cardiomyocytes in a paracrine manner; and their secreted factors such as CCL24, GAS6 or AREG potentiate neonatal cardiomyocyte proliferation. By comparing the regenerating P3 and non-regenerating P8 heart, there is a significant increase in the absolute number of intracardiac Treg but the whole transcriptomes of these Treg do not differ regardless of whether the neonatal heart regenerates. Furthermore, even adult Treg, given sufficient quantity, possess the same regenerative capability. Conclusion: Our results demonstrate a regenerative role of Treg in neonatal heart regeneration. Treg can directly facilitate cardiomyocyte proliferation in a paracrine manner.
Keywords: CD4+ regulatory T-cells; cardiac fibrosis; cardiomyocyte proliferation; heart regeneration; macrophages; single-cell RNA-seq.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

Similar articles
-
Specific ablation of CD4+ T-cells promotes heart regeneration in juvenile mice.Theranostics. 2020 Jun 29;10(18):8018-8035. doi: 10.7150/thno.42943. eCollection 2020. Theranostics. 2020. PMID: 32724455 Free PMC article.
-
Tissue extracellular vesicles suppress neonatal cardiac regeneration: a Pak2-Erk1/2-mediated macrophage paracrine signaling.Acta Biochim Biophys Sin (Shanghai). 2025 Feb 14;57(6):968-980. doi: 10.3724/abbs.2024193. Acta Biochim Biophys Sin (Shanghai). 2025. PMID: 39949198 Free PMC article.
-
Mydgf promotes Cardiomyocyte proliferation and Neonatal Heart regeneration.Theranostics. 2020 Jul 11;10(20):9100-9112. doi: 10.7150/thno.44281. eCollection 2020. Theranostics. 2020. PMID: 32802181 Free PMC article.
-
Regulatory T cells and skeletal muscle regeneration.FEBS J. 2017 Feb;284(4):517-524. doi: 10.1111/febs.13827. Epub 2016 Aug 18. FEBS J. 2017. PMID: 27479876 Review.
-
Cell migration during heart regeneration in zebrafish.Dev Dyn. 2016 Jul;245(7):774-87. doi: 10.1002/dvdy.24411. Epub 2016 May 10. Dev Dyn. 2016. PMID: 27085002 Free PMC article. Review.
Cited by
-
Syngeneic Mesenchymal Stem Cells Reduce Immune Rejection After Induced Pluripotent Stem Cell-Derived Allogeneic Cardiomyocyte Transplantation.Sci Rep. 2020 Mar 12;10(1):4593. doi: 10.1038/s41598-020-58126-z. Sci Rep. 2020. PMID: 32165680 Free PMC article.
-
Immune landscape in rejection of renal transplantation revealed by high-throughput single-cell RNA sequencing.Front Cell Dev Biol. 2023 Jul 20;11:1208566. doi: 10.3389/fcell.2023.1208566. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37547477 Free PMC article.
-
Complex regulation of cardiac fibrosis: insights from immune cells and signaling pathways.J Transl Med. 2025 Feb 28;23(1):242. doi: 10.1186/s12967-025-06260-5. J Transl Med. 2025. PMID: 40022104 Free PMC article. Review.
-
Adult T-cells impair neonatal cardiac regeneration.Eur Heart J. 2022 Jul 21;43(28):2698-2709. doi: 10.1093/eurheartj/ehac153. Eur Heart J. 2022. PMID: 35417553 Free PMC article.
-
Adult Cardiomyocyte Proliferation: a New Insight for Myocardial Infarction Therapy.J Cardiovasc Transl Res. 2021 Jun;14(3):457-466. doi: 10.1007/s12265-020-10067-8. Epub 2020 Aug 20. J Cardiovasc Transl Res. 2021. PMID: 32820393 Review.
References
-
- Haubner BJ, Schneider J, Schweigmann U, Schuetz T, Dichtl W, Velik-Salchner C. et al. Functional Recovery of a Human Neonatal Heart After Severe Myocardial Infarction. Circ Res. 2016;118:216–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

