SIRT7 Regulates Lipopolysaccharide-Induced Inflammatory Injury by Suppressing the NF- κ B Signaling Pathway
- PMID: 31285783
- PMCID: PMC6594283
- DOI: 10.1155/2019/3187972
SIRT7 Regulates Lipopolysaccharide-Induced Inflammatory Injury by Suppressing the NF- κ B Signaling Pathway
Abstract
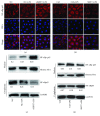
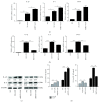
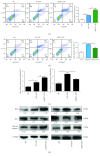
Mastitis has severely affected the cattle industry worldwide and has resulted in decreased dairy production and cattle reproduction. Although prevention and treatment methods have been implemented for decades, cattle mastitis is still an intractable disease. Sirtuin 7 (SIRT7) is an NAD+-dependent deacetylase that is involved in various biological processes, including ribosomal RNA synthesis and protein synthesis, DNA damage response, metabolism, and tumorigenesis. However, whether SIRT7 participates in inflammation remains unknown. Our results revealed that SIRT7 is downregulated in tissue samples from mastitic cattle. Therefore, we isolated dairy cow mammary epithelial cells (DCMECs) from breast tissues and developed an in vitro model of lipopolysaccharide- (LPS-) induced inflammation to examine SIRT7 function and its potential role in inflammation. We showed that SIRT7 was significantly downregulated in LPS-treated DCMECs. SIRT7 knockdown significantly increased the LPS-stimulated production of inflammatory mediators, like reactive oxygen and nitric oxide, and upregulated TAB1 and TLR4. In addition, SIRT7 knockdown significantly increased the phosphorylation of TAK1 and NF-κBp65 in LPS-treated DCMECs. Moreover, SIRT7 knockdown promoted the translocation of NF-κBp-p65 to the cell nucleus and then increased the secretion of inflammatory cytokines (IL-1β and IL-6). In contrast, SIRT7 overexpression had the opposite effects when compared to SIRT7 knockdown in LPS-treated DCMECs. In addition, SIRT7 overexpression attenuated LPS-induced DCMEC apoptosis. Taken together, our results indicate that SIRT7 can suppress LPS-induced inflammation and apoptosis via the NF-κB signaling pathway. Therefore, SIRT7 may be considered as a potential pharmacological target for clinical mastitis therapy.
Figures




Similar articles
-
14-3-3γ Regulates Lipopolysaccharide-Induced Inflammatory Responses and Lactation in Dairy Cow Mammary Epithelial Cells by Inhibiting NF-κB and MAPKs and Up-Regulating mTOR Signaling.Int J Mol Sci. 2015 Jul 22;16(7):16622-41. doi: 10.3390/ijms160716622. Int J Mol Sci. 2015. PMID: 26204835 Free PMC article.
-
Nuciferine alleviates LPS-induced mastitis in mice via suppressing the TLR4-NF-κB signaling pathway.Inflamm Res. 2018 Dec;67(11-12):903-911. doi: 10.1007/s00011-018-1183-2. Epub 2018 Aug 25. Inflamm Res. 2018. PMID: 30145653
-
Indirubin Treatment of Lipopolysaccharide-Induced Mastitis in a Mouse Model and Activity in Mouse Mammary Epithelial Cells.Mediators Inflamm. 2017;2017:3082805. doi: 10.1155/2017/3082805. Epub 2017 Feb 1. Mediators Inflamm. 2017. PMID: 28255203 Free PMC article.
-
Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPARγ/NF-κB pathways in primary bovine mammary epithelial cells.Res Vet Sci. 2017 Jun;112:7-12. doi: 10.1016/j.rvsc.2016.12.011. Epub 2017 Jan 5. Res Vet Sci. 2017. PMID: 28095338 Review.
-
Inflammatory mediators in mastitis and lactation insufficiency.J Mammary Gland Biol Neoplasia. 2014 Jul;19(2):161-7. doi: 10.1007/s10911-014-9325-9. Epub 2014 Jun 25. J Mammary Gland Biol Neoplasia. 2014. PMID: 24961655 Review.
Cited by
-
Combination of resolvin E1 and lipoxin A4 promotes the resolution of pulpitis by inhibiting NF-κB activation through upregulating sirtuin 7 in dental pulp fibroblasts.Cell Prolif. 2022 May;55(5):e13227. doi: 10.1111/cpr.13227. Epub 2022 Apr 11. Cell Prolif. 2022. PMID: 35411569 Free PMC article.
-
The sirtuin family in health and disease.Signal Transduct Target Ther. 2022 Dec 29;7(1):402. doi: 10.1038/s41392-022-01257-8. Signal Transduct Target Ther. 2022. PMID: 36581622 Free PMC article. Review.
-
The effect of different sources of mesenchymal stem cells on microglia states.Front Aging Neurosci. 2023 Aug 24;15:1237532. doi: 10.3389/fnagi.2023.1237532. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37693651 Free PMC article. Review.
-
Integration of protein context improves protein-based COVID-19 patient stratification.Clin Proteomics. 2022 Aug 11;19(1):31. doi: 10.1186/s12014-022-09370-0. Clin Proteomics. 2022. PMID: 35953823 Free PMC article.
-
Nitric oxide inhibits endothelial cell apoptosis by inhibiting cysteine-dependent SOD1 monomerization.FEBS Open Bio. 2022 Feb;12(2):538-548. doi: 10.1002/2211-5463.13362. Epub 2022 Jan 11. FEBS Open Bio. 2022. PMID: 34986524 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

