Reinvestigating the early embryogenesis in the flatworm Maritigrella crozieri highlights the unique spiral cleavage program found in polyclad flatworms
- PMID: 31285819
- PMCID: PMC6588950
- DOI: 10.1186/s13227-019-0126-5
Reinvestigating the early embryogenesis in the flatworm Maritigrella crozieri highlights the unique spiral cleavage program found in polyclad flatworms
Abstract
Background: Spiral cleavage is a conserved, early developmental mode found in several phyla of Lophotrochozoans resulting in highly diverse adult body plans. While the cleavage pattern has clearly been broadly conserved, it has also undergone many modifications in various taxa. The precise mechanisms of how different adaptations have altered the ancestral spiral cleavage pattern are an important ongoing evolutionary question, and adequately answering this question requires obtaining a broad developmental knowledge of different spirally cleaving taxa. In flatworms (Platyhelminthes), the spiral cleavage program has been lost or severely modified in most taxa. Polyclad flatworms, however, have retained the pattern up to the 32-cell stage. Here we study early embryogenesis of the cotylean polyclad flatworm Maritigrella crozieri to investigate how closely this species follows the canonical spiral cleavage pattern and to discover any potential deviations from it.
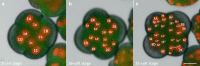
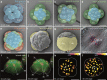
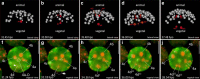
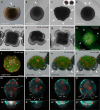
Results: Using live imaging recordings and 3D reconstructions of embryos, we give a detailed picture of the events that occur during spiral cleavage in M. crozieri. We suggest, contrary to previous observations, that the four-cell stage is a product of unequal cleavages. We show that that the formation of third and fourth micromere quartets is accompanied by strong blebbing events; blebbing also accompanies the formation of micromere 4d. We find an important deviation from the canonical pattern of cleavages with clear evidence that micromere 4d follows an atypical cleavage pattern, so far exclusively found in polyclad flatworms.
Conclusions: Our findings highlight that early development in M. crozieri deviates in several important aspects from the canonical spiral cleavage pattern. We suggest that some of our observations extend to polyclad flatworms in general as they have been described in both suborders of the Polycladida, the Cotylea and Acotylea.
Keywords: Blebbing; Evo-devo; Light-sheet microscopy; Live imaging; Polyclad flatworms; SPIM; Spiralians; Symmetry breaking; Turbellarians.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures




References
-
- Anderson DT. The embyonic and laval development of the turbellarian Notoplana australis (Schmarda, 1859) (Polycladida: Leptoplanidae) Mar Freshw Res. 1977;28:303–310. doi: 10.1071/MF9770303. - DOI
-
- Arnolds WJA, van den Biggelaar JAM, Verdonk NH. Spatial aspects of cell interactions involved in the determination of dorsoventral polarity in equally cleaving gastropods and regulative abilities of their embryos, as studied by micromere deletions in Lymnaea and Patella. Wilhelm Roux’s Arch Dev Biol. 1983;192:75–85. doi: 10.1007/BF00848483. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

