Clinical factors affecting the survival of patients diagnosed with non-small cell lung cancer and metastatic malignant pleural effusion, treated with hyperthermic intrathoracic chemotherapy or chemical talc pleurodesis: a monocentric, prospective, randomized trial
- PMID: 31285871
- PMCID: PMC6588789
- DOI: 10.21037/jtd.2019.05.25
Clinical factors affecting the survival of patients diagnosed with non-small cell lung cancer and metastatic malignant pleural effusion, treated with hyperthermic intrathoracic chemotherapy or chemical talc pleurodesis: a monocentric, prospective, randomized trial
Abstract
Background: There is a plethora of treatment algorithms for managing patients with malignant pleural effusions (MPEs), sharing many common points and principles. Our study aims to compare hyperthermic intrapleural chemotherapy (HITHOC) and talc pleurodesis (TALC), as treatment options for patients with non-small cell lung cancer (NSCLC) and metastatic MPE.
Methods: This prospective, randomized trial was conducted at a single thoracic surgery center, the "Theagenio" Cancer Institute, in Greece, under the identification code NCT01409551 and was completed. All 40 patients enrolled were adults with histologically proven metastatic, unilateral, MPE caused by NSCLC. Exclusion criteria included patients >80 years, trapped lung, and major comorbidities. Patients were randomly and equally assigned 1:1 to either HITHOC (group A) or TALC (group B) by video assisted thoracic surgery (VATS). The primary outcome was the median overall survival (OS) from trial intervention to death, while secondary outcome was the identification of clinical factors affecting the survival.
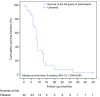
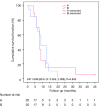
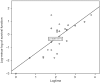
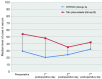
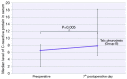
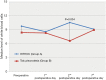
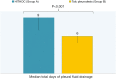
Results: The patients were followed up for 45 months. The OS of the full group was 8 months (95% CI: 7.046-8.954). Participants who underwent HITHOC had an OS of 8 months (95% CI: 7.141-8.859), whereas the participants of TALC had an OS of 9 months (95% CI: 7.546-10.454), with no significant difference between groups. Among fifty-four factors that were tested for their effects on survival, only TNM stage and creatinine values both preoperatively and 7 days postoperatively could be regarded as risk-factors for survival. Other recorded parameters, which had significant variance between the two groups, were urea levels, C-reactive protein, white blood cells and total in hospital length of stay (LOS).
Conclusions: Both HITHOC and TALC are equally effective and safe therapeutic options in treating patients with MPE and NSCLC with acceptable survival. The study revealed independent clinical risk factors influencing survival, which could be utilized as starting points for larger clinical studies.
Keywords: Pleurodesis; pleural effusion; malignant; carcinoma; non-small cell lung; hyperthermia.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
References
-
- Villena Garrido V, Cases Viedma E, Fernández Villar A, et al. Recommendations of diagnosis and treatment of pleural effusion. Update. Arch Bronconeumol 2014;50:235-49. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
