Arthroplasty studies with greater than 1000 participants: analysis of follow-up methods
- PMID: 31286051
- PMCID: PMC6588815
- DOI: 10.1016/j.artd.2019.03.006
Arthroplasty studies with greater than 1000 participants: analysis of follow-up methods
Abstract
Background: The use of patient-reported outcome measures (PROMs) has become a mainstay of orthopedic joint arthroplasty research. Large studies with >1000 participants are vital to orthopedic research, as they allow for comprehensive multivariable analysis. Achieving high follow-up rates minimizes potential response bias. Maintaining adequate follow-up rates becomes more challenging as sample size increases. We aimed to systematically review the present literature to determine the follow-up rates of large cohorts/registries of total joint arthroplasty patients and to identify factors associated with successful collection of PROMs.
Methods: A comprehensive literature search of PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Inclusion criteria were: ≥1000 participants, ≥6 months of postoperative follow-up, and use of validated PROMs postoperatively.
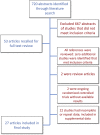
Results: Of 720 abstracts screened, 21 studies met inclusion criteria. Only 2 studies reported achieving a PROM follow-up rate ≥80%, but neither collected PROMs preoperatively. The median rate of follow-up was 70%, and the median number of patients was 2970. Only 38% (8 of 21) of studies collected baseline PROMs prior to surgery.
Conclusions: Very few studies in the present literature have collected validated PROMs on ≥1000 patients with ≥80% follow-up; these parameters are conducive to comprehensive multivariable analysis, while maintaining study validity and avoiding follow-up bias. Federal funding and a central coordinating site may be helpful in achieving follow-up in studies of this magnitude.
Level of evidence: Level III, systematic review of studies with Level of Evidence I-III.
Keywords: Arthroplasty; Hip; Knee; Patient-reported outcomes.
Figures
Similar articles
-
PROMIS Versus Legacy Patient-Reported Outcome Measures for Sports Medicine Patients Undergoing Arthroscopic Knee, Shoulder, and Hip Interventions: A Systematic Review.Iowa Orthop J. 2021 Dec;41(2):58-71. Iowa Orthop J. 2021. PMID: 34924871 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Current Trends in Patient-Reported Outcome Measures in Total Joint Arthroplasty: A Study of 4 Major Orthopaedic Journals.J Arthroplasty. 2018 Nov;33(11):3416-3421. doi: 10.1016/j.arth.2018.06.034. Epub 2018 Jul 3. J Arthroplasty. 2018. PMID: 30057269 Review.
-
Patient-reported outcome measures (PROMs) after elective hip, knee and shoulder arthroplasty: protocol for a prospective cohort study.BMC Musculoskelet Disord. 2019 Aug 15;20(1):374. doi: 10.1186/s12891-019-2745-3. BMC Musculoskelet Disord. 2019. PMID: 31416443 Free PMC article.
-
Substantial Loss to Follow-Up and Missing Data in National Arthroscopy Registries: A Systematic Review.Arthroscopy. 2021 Feb;37(2):761-770.e3. doi: 10.1016/j.arthro.2020.08.007. Epub 2020 Aug 22. Arthroscopy. 2021. PMID: 32835814
Cited by
-
Agreement between the American Shoulder and Elbow Surgeons Society Standardized Shoulder Assessment score (ASES) and the Oxford Shoulder Score (OSS) in patients presenting with shoulder pathology: A cohort analysis of the Clinical Quality Registry for Outcomes in Shoulder and Elbow Pathology (CROSEP) registry.Shoulder Elbow. 2022 Dec;14(6):682-691. doi: 10.1177/17585732211056073. Epub 2021 Dec 16. Shoulder Elbow. 2022. PMID: 36479016 Free PMC article.
-
Standardizing Muscle Strength Measurement in Femoroacetabular Impingement Syndrome: Response.Sports Health. 2021 Jul-Aug;13(4):404. doi: 10.1177/1941738120977419. Epub 2020 Nov 24. Sports Health. 2021. PMID: 33231501 Free PMC article. No abstract available.
-
Changes Over a Decade in Patient-Reported Outcome Measures and Minimal Clinically Important Difference Reporting in Total Joint Arthroplasty.Arthroplast Today. 2023 Mar 6;20:101096. doi: 10.1016/j.artd.2023.101096. eCollection 2023 Apr. Arthroplast Today. 2023. PMID: 36923058 Free PMC article.
-
Young or Old Age and Non-White Race Are Associated With Poor Patient-Reported Outcome Measure Response Compliance After Orthopaedic Surgery.Arthrosc Sports Med Rehabil. 2023 Nov 11;5(6):100817. doi: 10.1016/j.asmr.2023.100817. eCollection 2023 Dec. Arthrosc Sports Med Rehabil. 2023. PMID: 38023444 Free PMC article. Review.
-
An update on joint-specific outcome measures in total hip replacement.Reumatologia. 2020;58(2):107-115. doi: 10.5114/reum.2020.95366. Epub 2020 Apr 30. Reumatologia. 2020. PMID: 32476684 Free PMC article. Review.
References
-
- Rolfson O., Bohm E., Franklin P. Patient-reported outcome measures in arthroplasty registries: report of the patient-reported outcome measures working group of the International Society of Arthroplasty Registries Part II. Recommendations for selection, administration, and analysis. Acta Orthop. 2016;87(Supp1.):9. - PMC - PubMed
-
- Porter M.E. What is value in health care? N Engl J Med. 2010;363(26):2477. - PubMed
-
- Rolfson O., Eresian Chenok K., Bohm E. Patient-reported outcome measures in arthroplasty registries: report of the patient-reported outcome measures working group of the International Society of Arthroplasty Registries Part I. Overview and rationale for patient-reported outcome measures. Acta Orthop. 2016;87(Supp1.):3. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous