Alcohol sedation in adult Drosophila is regulated by Cysteine proteinase-1 in cortex glia
- PMID: 31286069
- PMCID: PMC6610072
- DOI: 10.1038/s42003-019-0492-5
Alcohol sedation in adult Drosophila is regulated by Cysteine proteinase-1 in cortex glia
Abstract
Although numerous studies have demonstrated that neuronal mechanisms regulate alcohol-related behaviors, very few have investigated the direct role of glia in behavioral responses to alcohol. The results described here begin to fill this gap in the alcohol behavior and gliobiology fields. Since Drosophila exhibit conserved behavioral responses to alcohol and their CNS glia are similar to mammalian CNS glia, we used Drosophila to begin exploring the role of glia in alcohol behavior. We found that knockdown of Cysteine proteinase-1 (Cp1) in glia increased Drosophila alcohol sedation and that this effect was specific to cortex glia and adulthood. These data implicate Cp1 and cortex glia in alcohol-related behaviors. Cortex glia are functionally homologous to mammalian astrocytes and Cp1 is orthologous to mammalian Cathepsin L. Our studies raise the possibility that cathepsins may influence behavioral responses to alcohol in mammals via roles in astrocytes.
Keywords: Behavioural genetics; Glial biology.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
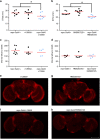
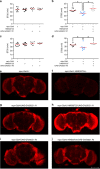
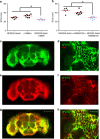

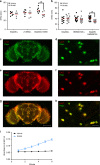
Similar articles
-
Tyramine synthesis, vesicular packaging, and the SNARE complex function coordinately in astrocytes to regulate Drosophila alcohol sedation.Addict Biol. 2021 Jul;26(4):e13019. doi: 10.1111/adb.13019. Epub 2021 Feb 3. Addict Biol. 2021. PMID: 33538092 Free PMC article.
-
The Unique Dopamine/Ecdysteroid Receptor Modulates Ethanol-Induced Sedation in Drosophila.J Neurosci. 2016 Apr 20;36(16):4647-57. doi: 10.1523/JNEUROSCI.3774-15.2016. J Neurosci. 2016. PMID: 27098705 Free PMC article.
-
The secreted neurotrophin Spätzle 3 promotes glial morphogenesis and supports neuronal survival and function.Genes Dev. 2017 Oct 15;31(20):2023-2038. doi: 10.1101/gad.305888.117. Epub 2017 Nov 14. Genes Dev. 2017. PMID: 29138279 Free PMC article.
-
Glial Regulation of Circuit Wiring, Firing, and Expiring in the Drosophila Central Nervous System.Cold Spring Harb Perspect Biol. 2024 Dec 2;16(12):a041347. doi: 10.1101/cshperspect.a041347. Cold Spring Harb Perspect Biol. 2024. PMID: 38565270 Free PMC article. Review.
-
Drosophila Central Nervous System Glia.Cold Spring Harb Perspect Biol. 2015 Feb 26;7(11):a020552. doi: 10.1101/cshperspect.a020552. Cold Spring Harb Perspect Biol. 2015. PMID: 25722465 Free PMC article. Review.
Cited by
-
Flying Together: Drosophila as a Tool to Understand the Genetics of Human Alcoholism.Int J Mol Sci. 2020 Sep 11;21(18):6649. doi: 10.3390/ijms21186649. Int J Mol Sci. 2020. PMID: 32932795 Free PMC article. Review.
-
Tyramine synthesis, vesicular packaging, and the SNARE complex function coordinately in astrocytes to regulate Drosophila alcohol sedation.Addict Biol. 2021 Jul;26(4):e13019. doi: 10.1111/adb.13019. Epub 2021 Feb 3. Addict Biol. 2021. PMID: 33538092 Free PMC article.
-
Cortical astrocytes regulate ethanol consumption and intoxication in mice.Neuropsychopharmacology. 2021 Feb;46(3):500-508. doi: 10.1038/s41386-020-0721-0. Epub 2020 May 28. Neuropsychopharmacology. 2021. PMID: 32464636 Free PMC article.
-
Identification of additional dye tracers for measuring solid food intake and food preference via consumption-excretion in Drosophila.Sci Rep. 2022 Apr 13;12(1):6201. doi: 10.1038/s41598-022-10252-6. Sci Rep. 2022. PMID: 35418664 Free PMC article.
-
The foraging gene affects alcohol sensitivity, metabolism and memory in Drosophila.J Neurogenet. 2021 Sep;35(3):236-248. doi: 10.1080/01677063.2021.1931178. Epub 2021 Jun 7. J Neurogenet. 2021. PMID: 34092172 Free PMC article.
References
-
- American Psychiatric, A. Diagnostic and Statistical Manual of Mental Disorders. 5 edn, (American Psychiatric Publishing, Washington, D.C., 2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

