HAND1 loss-of-function within the embryonic myocardium reveals survivable congenital cardiac defects and adult heart failure
- PMID: 31286141
- PMCID: PMC7252443
- DOI: 10.1093/cvr/cvz182
HAND1 loss-of-function within the embryonic myocardium reveals survivable congenital cardiac defects and adult heart failure
Abstract
Aims: To examine the role of the basic Helix-loop-Helix (bHLH) transcription factor HAND1 in embryonic and adult myocardium.
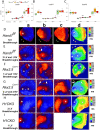
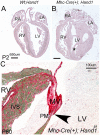
Methods and results: Hand1 is expressed within the cardiomyocytes of the left ventricle (LV) and myocardial cuff between embryonic days (E) 9.5-13.5. Hand gene dosage plays an important role in ventricular morphology and the contribution of Hand1 to congenital heart defects requires further interrogation. Conditional ablation of Hand1 was carried out using either Nkx2.5 knockin Cre (Nkx2.5Cre) or α-myosin heavy chain Cre (αMhc-Cre) driver. Interrogation of transcriptome data via ingenuity pathway analysis reveals several gene regulatory pathways disrupted including translation and cardiac hypertrophy-related pathways. Embryo and adult hearts were subjected to histological, functional, and molecular analyses. Myocardial deletion of Hand1 results in morphological defects that include cardiac conduction system defects, survivable interventricular septal defects, and abnormal LV papillary muscles (PMs). Resulting Hand1 conditional mutants are born at Mendelian frequencies; but the morphological alterations acquired during cardiac development result in, the mice developing diastolic heart failure.
Conclusion: Collectively, these data reveal that HAND1 contributes to the morphogenic patterning and maturation of cardiomyocytes during embryogenesis and although survivable, indicates a role for Hand1 within the developing conduction system and PM development.
Keywords: HAND1; Transcription; Cardiac development; Heart failure with preserved ejection fraction; Mitral arcade; Right bundle branch block.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures





Comment in
-
Hearts and Hands: the good, the bad, and the ugly.Cardiovasc Res. 2020 Mar 1;116(3):470-472. doi: 10.1093/cvr/cvz273. Cardiovasc Res. 2020. PMID: 31702005 Free PMC article. No abstract available.
References
-
- Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, McGee G, Sable CA, Srivastava D, Webb CL; American Heart Association Congenital Cardiac Defects Committee; Council on Cardiovascular Disease in the Young. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007;115:3015–3038. - PubMed
-
- Gittenberger-de Groot AC, Blom NM, Aoyama N, Sucov H, Wenink AC, Poelmann RE.. The role of neural crest and epicardium-derived cells in conduction system formation. Novartis Found Symp 2008;250:125–134. discussion 134-141. - PubMed
-
- Rochais F, Mesbah K, Kelly RG.. Signaling pathways controlling second heart field development. Circ Res 2009;104:933–942. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

