Pre-operative Brain Imaging Using Functional Near-Infrared Spectroscopy Helps Predict Cochlear Implant Outcome in Deaf Adults
- PMID: 31286300
- PMCID: PMC6797684
- DOI: 10.1007/s10162-019-00729-z
Pre-operative Brain Imaging Using Functional Near-Infrared Spectroscopy Helps Predict Cochlear Implant Outcome in Deaf Adults
Abstract
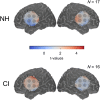
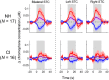
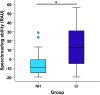
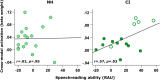
Currently, it is not possible to accurately predict how well a deaf individual will be able to understand speech when hearing is (re)introduced via a cochlear implant. Differences in brain organisation following deafness are thought to contribute to variability in speech understanding with a cochlear implant and may offer unique insights that could help to more reliably predict outcomes. An emerging optical neuroimaging technique, functional near-infrared spectroscopy (fNIRS), was used to determine whether a pre-operative measure of brain activation could explain variability in cochlear implant (CI) outcomes and offer additional prognostic value above that provided by known clinical characteristics. Cross-modal activation to visual speech was measured in bilateral superior temporal cortex of pre- and post-lingually deaf adults before cochlear implantation. Behavioural measures of auditory speech understanding were obtained in the same individuals following 6 months of cochlear implant use. The results showed that stronger pre-operative cross-modal activation of auditory brain regions by visual speech was predictive of poorer auditory speech understanding after implantation. Further investigation suggested that this relationship may have been driven primarily by the inclusion of, and group differences between, pre- and post-lingually deaf individuals. Nonetheless, pre-operative cortical imaging provided additional prognostic value above that of influential clinical characteristics, including the age-at-onset and duration of auditory deprivation, suggesting that objectively assessing the physiological status of the brain using fNIRS imaging pre-operatively may support more accurate prediction of individual CI outcomes. Whilst activation of auditory brain regions by visual speech prior to implantation was related to the CI user's clinical history of deafness, activation to visual speech did not relate to the future ability of these brain regions to respond to auditory speech stimulation with a CI. Greater pre-operative activation of left superior temporal cortex by visual speech was associated with enhanced speechreading abilities, suggesting that visual speech processing may help to maintain left temporal lobe specialisation for language processing during periods of profound deafness.
Keywords: cochlear implantation; cross-modal plasticity; functional near-infrared spectroscopy; prognostic imaging; speechreading; superior temporal cortex.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures




References
-
- Anderson CA (2016) Cortical predictors and correlates of cochlear implant outcome: a longitudinal study using functional near-infrared spectroscopy. PhD thesis, University of Nottingham. http://eprints.nottingham.ac.uk/id/eprint/37948
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

