Bromine-Promoted Glycosidation of Conformationally Superarmed Thioglycosides
- PMID: 31286579
- PMCID: PMC6742554
- DOI: 10.1002/chem.201901969
Bromine-Promoted Glycosidation of Conformationally Superarmed Thioglycosides
Abstract
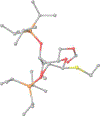
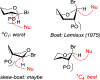
Presented herein is a study of the conformation and reactivity of highly reactive thioglycoside donors. The structural studies have been conducted using NMR spectroscopy and computational methods. The reactivity of these donors has been investigated in bromine-promoted glycosylations of aliphatic and sugar alcohols. Swift reaction times, high yields, and respectable 1,2-cis stereoselectivity were observed in a majority of these glycosylations.
Keywords: carbohydrate chemistry; glycosylation; stereocontrolled reactions; synthesis.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Panza M, Pistorio SG, Stine KJ, Demchenko AV, Chem. Rev 2018, 118, 8105–8150; - PMC - PubMed
- Nielsen MM, Pedersen CM, Chem. Rev 2018, 118, 8285–8358; - PubMed
- Kulkarni SS, Wang CC, Sabbavarapu NM, Podilapu AR, Liao PH, Hung SC, Chem. Rev 2018, 118, 8025–8104; - PubMed
- Bennett CS, Galan MC, Chem. Rev 2018, 118, 7931–7985; - PMC - PubMed
- Adero PO, Amarasekara H, Wen P, Bohe L, Crich D, Chem. Rev 2018, 118, 8242–8284. - PMC - PubMed
-
- Romero JAC, Tabacco SA, Woerpel KA, J. Am. Chem. Soc 2000, 122, 168–169;
- Ayala L, Lucero CG, Romero JAC, Tabacco SA, Woerpel KA, J. Am. Chem. Soc 2003, 125, 15521–15528; - PubMed
- Shenoy SR, Woerpel KA, Org. Lett 2005, 7, 1157–1160; - PubMed
- Baghdasarian G, Woerpel KA, J. Org. Chem 2006, 71, 6851–6858; - PubMed
- Billings SB, Woerpel KA, J. Org. Chem 2006, 71, 5171–5178; - PubMed
- Yang MT, Woerpel KA, J. Org. Chem 2009, 74, 545–553; - PMC - PubMed
- Beaver MG, Woerpel KA, J. Org. Chem 2010, 75, 1107–1118; - PMC - PubMed
- Garcia A, Sanzone JR, Woerpel KA, Angew. Chem. Int. Ed. Engl 2015, 54, 12087–12090. - PMC - PubMed
-
- Mootoo DR, Konradsson P, Udodong U, Fraser-Reid B, J. Am. Chem. Soc 1988, 110, 5583–5584;
- Fraser-Reid B, Udodong UE, Wu ZF, Ottosson H, Merritt JR, Rao CS, Roberts C, Madsen R, Synlett 1992, 927–942 and references therein.

