Thermal biology of mosquito-borne disease
- PMID: 31286630
- PMCID: PMC6744319
- DOI: 10.1111/ele.13335
Thermal biology of mosquito-borne disease
Abstract
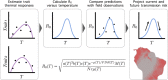
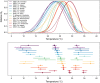
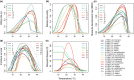
Mosquito-borne diseases cause a major burden of disease worldwide. The vital rates of these ectothermic vectors and parasites respond strongly and nonlinearly to temperature and therefore to climate change. Here, we review how trait-based approaches can synthesise and mechanistically predict the temperature dependence of transmission across vectors, pathogens, and environments. We present 11 pathogens transmitted by 15 different mosquito species - including globally important diseases like malaria, dengue, and Zika - synthesised from previously published studies. Transmission varied strongly and unimodally with temperature, peaking at 23-29ºC and declining to zero below 9-23ºC and above 32-38ºC. Different traits restricted transmission at low versus high temperatures, and temperature effects on transmission varied by both mosquito and parasite species. Temperate pathogens exhibit broader thermal ranges and cooler thermal minima and optima than tropical pathogens. Among tropical pathogens, malaria and Ross River virus had lower thermal optima (25-26ºC) while dengue and Zika viruses had the highest (29ºC) thermal optima. We expect warming to increase transmission below thermal optima but decrease transmission above optima. Key directions for future work include linking mechanistic models to field transmission, combining temperature effects with control measures, incorporating trait variation and temperature variation, and investigating climate adaptation and migration.
Keywords: Arbovirus; Ross River virus; West Nile virus; Zika virus; climate change; dengue virus; malaria; mosquito; temperature; thermal performance curve.
© 2019 The Authors Ecology Letters published by CNRS and John Wiley & Sons Ltd.
Figures


References
-
- Akey, D.H. , Potter, H.W. & Jone, R.H. (1978). Effects of rearing temperature and larval density on longevity, size, and fecundity in the biting gnat Culicoides variipennis. Ann. Entomol. Soc. Am., 71, 411–418.
-
- Alsan, M. (2015). The effect of the TseTse fly on African development. Am. Econ. Rev., 105, 382–410.
-
- Amarasekare, P. & Savage, V. (2012). A framework for elucidating the temperature dependence of fitness. Am. Nat., 179, 178–191. - PubMed
-
- Angilletta, M.J. (2009). Thermal Adaptation: A Theoretical and Empirical Synthesis. Oxford, UK:Oxford University Press.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

