The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy
- PMID: 31286691
- PMCID: PMC6818463
- DOI: 10.1002/jcsm.12461
The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is a particularly lethal malignancy partly due to frequent, severe cachexia. Serum activin correlates with cachexia and mortality, while exogenous activin causes cachexia in mice.
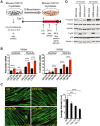
Methods: Isoform-specific activin expression and activities were queried in human and murine tumours and PDAC models. Activin inhibition was by administration of soluble activin type IIB receptor (ACVR2B/Fc) and by use of skeletal muscle specific dominant negative ACVR2B expressing transgenic mice. Feed-forward activin expression and muscle wasting activity were tested in vivo and in vitro on myotubes.
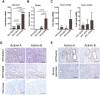
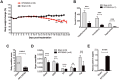
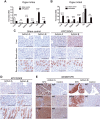
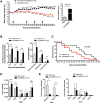
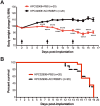
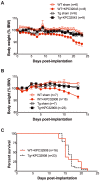
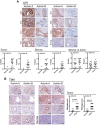
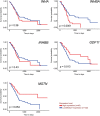
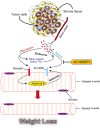
Results: Murine PDAC tumour-derived cell lines expressed activin-βA but not activin-βB. Cachexia severity increased with activin expression. Orthotopic PDAC tumours expressed activins, induced activin expression by distant organs, and produced elevated serum activins. Soluble factors from PDAC elicited activin because conditioned medium from PDAC cells induced activin expression, activation of p38 MAP kinase, and atrophy of myotubes. The activin trap ACVR2B/Fc reduced tumour growth, prevented weight loss and muscle wasting, and prolonged survival in mice with orthotopic tumours made from activin-low cell lines. ACVR2B/Fc also reduced cachexia in mice with activin-high tumours. Activin inhibition did not affect activin expression in organs. Hypermuscular mice expressing dominant negative ACVR2B in muscle were protected for weight loss but not mortality when implanted with orthotopic tumours. Human tumours displayed staining for activin, and expression of the gene encoding activin-βA (INHBA) correlated with mortality in patients with PDAC, while INHBB and other related factors did not.
Conclusions: Pancreatic adenocarcinoma tumours are a source of activin and elicit a systemic activin response in hosts. Human tumours express activins and related factors, while mortality correlates with tumour activin A expression. PDAC tumours also choreograph a systemic activin response that induces organ-specific and gene-specific expression of activin isoforms and muscle wasting. Systemic blockade of activin signalling could preserve muscle and prolong survival, while skeletal muscle-specific activin blockade was only protective for weight loss. Our findings suggest the potential and need for gene-specific and organ-specific interventions. Finally, development of more effective cancer cachexia therapy might require identifying agents that effectively and/or selectively inhibit autocrine vs. paracrine activin signalling.
Keywords: Activin receptor type IIb; Activins; Cachexia; Pancreatic cancer; Weight loss.
© 2019 The Authors Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Blum D, Stene GB, Solheim TS, Fayers P, Hjermstad MJ, Baracos VE, et al. Validation of the consensus‐definition for cancer cachexia and evaluation of a classification model—a study based on data from an international multicentre project (epcrc‐csa). Ann Oncol 2014;25:1635–1642. - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. - PubMed
-
- Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer‐associated cachexia. Nat Rev Dis Primers 2018;4:17105. - PubMed
-
- Werner S, Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev 2006;17:157–171. - PubMed
-
- Hedger MP, Winnall WR. Regulation of activin and inhibin in the adult testis and the evidence for functional roles in spermatogenesis and immunoregulation. Mol Cell Endocrinol 2012;359:30–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM092758/GM/NIGMS NIH HHS/United States
- R01 CA122596/CA/NCI NIH HHS/United States
- P30CA082709/GF/NIH HHS/United States
- P30 CA082709/CA/NCI NIH HHS/United States
- R01CA122596/GF/NIH HHS/United States
- R01DK096167/GF/NIH HHS/United States
- R01 DK096167/DK/NIDDK NIH HHS/United States
- P30 CA023168/CA/NCI NIH HHS/United States
- R01 CA194593/CA/NCI NIH HHS/United States
- R01GM092758/GF/NIH HHS/United States
- P30CA023168/BC/NCI NIH HHS/United States
- P30CA082709/BC/NCI NIH HHS/United States
- I01 BX004177/BX/BLRD VA/United States
- R01CA194593/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

