Examining the Impact of Refractory Myasthenia Gravis on Healthcare Resource Utilization in the United States: Analysis of a Myasthenia Gravis Foundation of America Patient Registry Sample
- PMID: 31286711
- PMCID: PMC6620464
- DOI: 10.3988/jcn.2019.15.3.376
Examining the Impact of Refractory Myasthenia Gravis on Healthcare Resource Utilization in the United States: Analysis of a Myasthenia Gravis Foundation of America Patient Registry Sample
Abstract
Background and purpose: Patients with refractory myasthenia gravis (MG) experience ongoing disease burden that might be reflected in their healthcare utilization. Here we examine the impact of refractory MG on healthcare utilization.
Methods: The 825 included participants were aged 18-64 years, enrolled in the Myasthenia Gravis Foundation of America Patient Registry between July 2013 and February 2018, and had been diagnosed with MG ≥2 years previously.
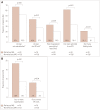
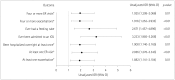
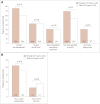
Results: Participants comprised 76 (9.2%) with refractory MG and 749 (90.8%) with nonrefractory MG. During the 6 months before enrollment, participants with refractory MG were significantly more likely than those with nonrefractory MG to have experienced at least one exacerbation [67.1% vs. 52.0%, respectively, p=0.01; odds ratio (OR)=1.882, 95% confidence interval (CI)=1.141-3.104], visited an emergency room at least once [43.4% vs. 27.1%, p<0.01; OR=2.065, 95% CI=1.276-3.343], been hospitalized overnight at least once (32.9% vs. 20.5%, p=0.01; OR=1.900, 95% CI=1.140-3.165), ever been admitted to an intensive care unit (ICU) (61.8% vs. 33.4%, p<0.01; OR=3.233, 95% CI=1.985-5.266), or ever required a feeding tube (21.1% vs. 9.1%, p<0.01; OR=2.671, 95% CI=1.457-4.896). A total of 75.8% younger females with refractory disease (<51 years, n=33) experienced at least one exacerbation, 69.7% had been admitted to an ICU, and 30.3% had required a feeding tube. For older females with refractory disease (≥51 years, n=33), 60.6%, 54.6%, and 6.1% experienced these outcomes, respectively (between-group differences were not significant).
Conclusions: Refractory MG is associated with higher disease burden and healthcare utilization than nonrefractory MG.
Keywords: disease exacerbations; healthcare resource utilization; myasthenia gravis; refractory disease.
Copyright © 2019 Korean Neurological Association.
Conflict of interest statement
Linda A. Harris is employed by Alexion Pharmaceuticals, Inc.; Gary Cutter, Haichang Xin, and Inmaculada B. Aban are employed by the University of Alabama at Birmingham, which received financial support from Alexion for this study. Gary Cutter is also President of Pythagoras, Inc., a private consulting company located in Birmingham, AL, USA, and is Professor of Biostatistics at the School of Public Health at the University of Alabama at Birmingham. Gary Cutter has served as a member of consulting or advisory boards (Argenx, Atara Biotherapeutics, Axon, Biogen, BrainStorm Cell Therapeutics, Charleston Laboratories, Click Therapeutics, Genentech, Genzyme, GW Pharmaceuticals, Klein Buendel, MedDay Pharmaceuticals, MedImmune, Novartis, Roche, SciFluor Life Sciences, Somahlution, Teva, TG Therapeutics, and UT Houston), and data and safety monitoring boards [AMO Pharma, BioLineRx, Hisun USA, and Horizon Pharma, Merck, Merck/Pfizer, National Heart, Lung, and Blood Institute (NHLBI; Protocol Review Committee), Neurim Pharmaceuticals, National Institute of Child Health and Human Development (NICHD; OPRU Oversight Committee), Novartis, OPKO Biologics, Orphazyme, Reata Pharmaceuticals, Receptos/Celgene, Sanofi-Aventis, and Teva].
Figures

References
-
- Heldal AT, Owe JF, Gilhus NE, Romi F. Seropositive myasthenia gravis: a nationwide epidemiologic study. Neurology. 2009;73:150–151. - PubMed
-
- Fang F, Sveinsson O, Thormar G, Granqvist M, Askling J, Lundberg IE, et al. The autoimmune spectrum of myasthenia gravis: a Swedish population-based study. J Intern Med. 2015;277:594–604. - PubMed
-
- Cetin H, Fülöp G, Zach H, Auff E, Zimprich F. Epidemiology of myasthenia gravis in Austria: rising prevalence in an ageing society. Wien Klin Wochenschr. 2012;124:763–768. - PubMed
-
- Murai H, Yamashita N, Watanabe M, Nomura Y, Motomura M, Yoshikawa H, et al. Characteristics of myasthenia gravis according to onset-age: Japanese nationwide survey. J Neurol Sci. 2011;305:97–102. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

