Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhesion in weaned piglets
- PMID: 31286936
- PMCID: PMC6615277
- DOI: 10.1186/s12917-019-1958-x
Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhesion in weaned piglets
Abstract
Background: Enterotoxigenic Escherichia coli K88 (E. coli K88) are considered as a major cause of diarrhea and death in newly weaned piglets. Oral passive immunization with chicken egg yolk immunoglobulins (IgY) have attracted considerable attention for treatment of gastrointestinal infection due to its high specificity. In this study it was estimated the protective effect of anti-K88 fimbriae IgY against E. coli K88 adhesion to piglet intestinal mucus in vitro and to investigate the potential use of IgY for controlling E. coli-induced diarrhea in weaned piglets in vivo.
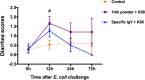
Results: E. coli K88 was incubated with IgY for 24 h, and the bacterial growth profiles showed that specific IgY with a concentration higher than 5 mg/mL was observed to significantly inhibit the growth of E. coli K88 compared to nonspecific yolk powder in a liquid medium. Moreover, pretreatment with 50 mg/mL of IgY was found to significantly decrease the adhesion ability of E. coli K88 to porcine jejunal and ileal mucus, further supported by the observations from our immunofluorescence microscopic analysis. In vivo, administration of IgY successfully protected piglets from diarrhea caused by E. coli K88 challenge. Additionally, IgY treatment efficiently alleviated E. coli-induced intestinal inflammation in piglets as the gene expression levels of inflammatory cytokines TNF-α, IL-22, IL-6 and IL-1β in IgY-treated piglets remained unchanged after E. coli K88 infection. Furthermore, IgY significantly prevented E. coli K88 adhering to the jejunal and ileal mucosa of piglets with E. coli infection and significantly decreased E. coli and enterotoxin expression in colonic contents.
Conclusion: Outcome of the study demonstrated that IgY against the fimbrial antigen K88 was able to significantly inhibit the growth of E. coli K88, block the binding of E. coli to small intestinal mucus, and protect piglets from E. coli-induced diarrhea. These results indicate that passive immunization with IgY may be useful to prevent bacterial colonization and to control enteric diseases due to E. coli infection. The study has great clinical implication to provide alternative therapy to antibiotics in E coli induced diarrhea.
Keywords: Bacteria adhesion; Diarrhea; Egg yolk immunoglobulins (IgY); Escherichia coli K88; Piglets.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
A Combination of Egg Yolk IgY and Phosvitin Inhibits the Growth of Enterotoxigenic Escherichia coli K88 and K99.Curr Pharm Biotechnol. 2017;18(5):400-409. doi: 10.2174/1389201018666170425120036. Curr Pharm Biotechnol. 2017. PMID: 28443510
-
Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets.FEMS Immunol Med Microbiol. 1999 Apr;23(4):283-8. doi: 10.1111/j.1574-695X.1999.tb01249.x. FEMS Immunol Med Microbiol. 1999. PMID: 10225287
-
Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets.Infect Immun. 1992 Mar;60(3):998-1007. doi: 10.1128/iai.60.3.998-1007.1992. Infect Immun. 1992. PMID: 1347289 Free PMC article.
-
Intestinal receptors for adhesive fimbriae of enterotoxigenic Escherichia coli (ETEC) K88 in swine--a review.Appl Microbiol Biotechnol. 2000 Sep;54(3):311-8. doi: 10.1007/s002530000404. Appl Microbiol Biotechnol. 2000. PMID: 11030565 Review.
-
Passive immunotherapy using chicken egg yolk antibody (IgY) against diarrheagenic E. coli: A systematic review and meta-analysis.Int Immunopharmacol. 2022 Jan;102:108381. doi: 10.1016/j.intimp.2021.108381. Epub 2021 Nov 20. Int Immunopharmacol. 2022. PMID: 34810126
Cited by
-
Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli.Open Life Sci. 2023 Dec 31;18(1):20220804. doi: 10.1515/biol-2022-0804. eCollection 2023. Open Life Sci. 2023. PMID: 38196514 Free PMC article.
-
Effects of Rhamnolipids on Growth Performance, Immune Function, and Cecal Microflora in Linnan Yellow Broilers Challenged with Lipopolysaccharides.Antibiotics (Basel). 2021 Jul 24;10(8):905. doi: 10.3390/antibiotics10080905. Antibiotics (Basel). 2021. PMID: 34438955 Free PMC article.
-
Effects of rhamnolipids on growth performance and intestinal health parameters in Linnan yellow broilers.Poult Sci. 2021 Feb;100(2):810-819. doi: 10.1016/j.psj.2020.10.041. Epub 2020 Nov 2. Poult Sci. 2021. PMID: 33518135 Free PMC article.
-
The impact of porcine spray-dried plasma protein and dried egg protein harvested from hyper-immunized hens, provided in the presence or absence of subtherapeutic levels of antibiotics in the feed, on growth and indicators of intestinal function and physiology of nursery pigs.Transl Anim Sci. 2020 Jun 26;4(3):txaa095. doi: 10.1093/tas/txaa095. eCollection 2020 Jul. Transl Anim Sci. 2020. PMID: 32844150 Free PMC article.
-
Cellular effects of oral egg yolk immunoglobulin-based supplementation at birth on promoting growth and strengthening intestinal mucosal innate immunity in pre-weaned piglets.Front Vet Sci. 2025 Jul 16;12:1458279. doi: 10.3389/fvets.2025.1458279. eCollection 2025. Front Vet Sci. 2025. PMID: 40740302 Free PMC article.
References
-
- Waters J, Sellwood R. Proceedings of the 2nd World Congress on Genetics Applied to Livestock Production. Madrid: International committee for world congress on genetics applied to livestock production; 1982. Aspects of genetic resistance to K88 E. coli in pigs; p. 8.
MeSH terms
Substances
Grants and funding
- QYZDY-SSW- SMC008/Key Programs of Frontier Scientific Research of the Chinese Academy of Sciences
- 31330075/National Natural Science Foundation of China
- 2017JJ1020/Natural Science Foundation of Hunan Province
- 2016RS3028/Huxiang Youth talent Support Program
- YESS20160086/Young Elite Scientists Sponsorship Program by CAST
LinkOut - more resources
Full Text Sources
Medical

