Weighing the evidence for pharmacological treatment interventions in mild COPD; a narrative perspective
- PMID: 31286970
- PMCID: PMC6615221
- DOI: 10.1186/s12931-019-1108-9
Weighing the evidence for pharmacological treatment interventions in mild COPD; a narrative perspective
Abstract
There is increasing focus on understanding the nature of chronic obstructive pulmonary disease (COPD) during the earlier stages. Mild COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage 1 or the now-withdrawn GOLD stage 0) represents an early stage of COPD that may progress to more severe disease. This review summarises the disease burden of patients with mild COPD and discusses the evidence for treatment intervention in this subgroup.Overall, patients with mild COPD suffer a substantial disease burden that includes persistent or potentially debilitating symptoms, increased risk of exacerbations, increased healthcare utilisation, reduced exercise tolerance and physical activity, and a higher rate of lung function decline versus controls. However, the evidence for treatment efficacy in these patients is limited due to their frequent exclusion from clinical trials. Careful assessment of disease burden and the rate of disease progression in individual patients, rather than a reliance on spirometry data, may identify patients who could benefit from earlier treatment intervention.
Keywords: Chronic obstructive pulmonary disease; Corticosteroid; Early intervention.
Conflict of interest statement
DS has received research, consulting and lecturing fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Genentech, GlaxoSmithKline, Glenmark, Menarini, Mundipharma, Novartis, Peptinnovate, Pfizer, Pulmatrix, Therevance, and Verona. AD has received research, consulting and lecturing fees from Boehringer Ingelheim (Canada), and Novartis Canada. JD has received research, consulting and lecturing fees from AstraZeneca, GSK, Mylan, Sunovion, and Theravance. EK has received research, consulting and lecturing fees from Amphastar, AstraZeneca, Boehringer Ingelheim, Crisor LLC Research, GlaxoSmithKline, Mylan, Novartis, Oriel, Pearl, Sunovion, Teva, and Theravance.
Figures
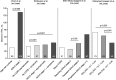
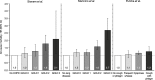
References
-
- Singh Dave, Agusti Alvar, Anzueto Antonio, Barnes Peter J., Bourbeau Jean, Celli Bartolome R., Criner Gerard J., Frith Peter, Halpin David M.G., Han Meilan, López Varela M. Victorina, Martinez Fernando, Montes de Oca Maria, Papi Alberto, Pavord Ian D., Roche Nicolas, Sin Donald D., Stockley Robert, Vestbo Jørgen, Wedzicha Jadwiga A., Vogelmeier Claus. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. European Respiratory Journal. 2019;53(5):1900164. doi: 10.1183/13993003.00164-2019. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

