Correlation between frataxin expression and contractility revealed by in vitro Friedreich's ataxia cardiac tissue models engineered from human pluripotent stem cells
- PMID: 31286988
- PMCID: PMC6615274
- DOI: 10.1186/s13287-019-1305-y
Correlation between frataxin expression and contractility revealed by in vitro Friedreich's ataxia cardiac tissue models engineered from human pluripotent stem cells
Abstract
Background: Friedreich's ataxia (FRDA) is an autosomal recessive disease caused by a non-coding mutation in the first intron of the frataxin (FXN) gene that suppresses its expression. Compensatory hypertrophic cardiomyopathy, dilated cardiomyopathy, and conduction system abnormalities in FRDA lead to cardiomyocyte (CM) death and fibrosis, consequently resulting in heart failure and arrhythmias. Murine models have been developed to study disease pathology in the past two decades; however, differences between human and mouse physiology and metabolism have limited the relevance of animal studies in cardiac disease conditions. To bridge this gap, we aimed to generate species-specific, functional in vitro experimental models of FRDA using 2-dimensional (2D) and 3-dimensional (3D) engineered cardiac tissues from FXN-deficient human pluripotent stem cell-derived ventricular cardiomyocytes (hPSC-hvCMs) and to compare their contractile and electrophysiological properties with healthy tissue constructs.
Methods: Healthy control and FRDA patient-specific hPSC-hvCMs were derived by directed differentiation using a small molecule-based protocol reported previously. We engineered the hvCMs into our established human ventricular cardiac tissue strip (hvCTS) and human ventricular cardiac anisotropic sheet (hvCAS) models, and functional assays were performed on days 7-17 post-tissue fabrication to assess the electrophysiology and contractility of FRDA patient-derived and FXN-knockdown engineered tissues, in comparison with healthy controls. To further validate the disease model, forced expression of FXN was induced in FXN-deficient tissues to test if disease phenotypes could be rescued.
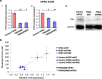
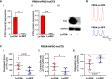
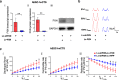
Results: Here, we report for the first time the generation of human engineered tissue models of FRDA cardiomyopathy from hPSCs: FXN-deficient hvCTS displayed attenuated developed forces (by 70-80%) compared to healthy controls. High-resolution optical mapping of hvCAS with reduced FXN expression also revealed electrophysiological defects consistent with clinical observations, including action potential duration prolongation and maximum capture frequency reduction. Interestingly, a clear positive correlation between FXN expression and contractility was observed (ρ > 0.9), and restoration of FXN protein levels by lentiviral transduction rescued contractility defects in FXN-deficient hvCTS.
Conclusions: We conclude that human-based in vitro cardiac tissue models of FRDA provide a translational, disease-relevant biomimetic platform for the evaluation of novel therapeutics and to provide insight into FRDA disease progression.
Conflict of interest statement
GW, KDC, CWC, and RAL hold equities in Novoheart whose value may potentially be affected by the publication of this manuscript. AOTW, MS, MZYC, WWT, BG, SYM, and DKL declared that they have no competing interests. AM and JFN were employees of Pfizer Inc. at the time this work was carried out.
Figures



Similar articles
-
Friedreich's ataxia induced pluripotent stem cell-derived cardiomyocytes display electrophysiological abnormalities and calcium handling deficiency.Aging (Albany NY). 2017 May 30;9(5):1440-1452. doi: 10.18632/aging.101247. Aging (Albany NY). 2017. PMID: 28562313 Free PMC article.
-
Excision of the expanded GAA repeats corrects cardiomyopathy phenotypes of iPSC-derived Friedreich's ataxia cardiomyocytes.Stem Cell Res. 2019 Oct;40:101529. doi: 10.1016/j.scr.2019.101529. Epub 2019 Aug 7. Stem Cell Res. 2019. PMID: 31446150 Free PMC article.
-
Progressive mitochondrial protein lysine acetylation and heart failure in a model of Friedreich's ataxia cardiomyopathy.PLoS One. 2017 May 25;12(5):e0178354. doi: 10.1371/journal.pone.0178354. eCollection 2017. PLoS One. 2017. PMID: 28542596 Free PMC article.
-
Biochemistry of cardiomyopathy in the mitochondrial disease Friedreich's ataxia.Biochem J. 2013 Aug 1;453(3):321-36. doi: 10.1042/BJ20130079. Biochem J. 2013. PMID: 23849057 Review.
-
Using human pluripotent stem cells to study Friedreich ataxia cardiomyopathy.Int J Cardiol. 2016 Jun 1;212:37-43. doi: 10.1016/j.ijcard.2016.03.040. Epub 2016 Mar 21. Int J Cardiol. 2016. PMID: 27019046 Review.
Cited by
-
Adult human cardiac stem cell supplementation effectively increases contractile function and maturation in human engineered cardiac tissues.Stem Cell Res Ther. 2019 Dec 4;10(1):373. doi: 10.1186/s13287-019-1486-4. Stem Cell Res Ther. 2019. PMID: 31801634 Free PMC article.
-
Machine Learning Techniques to Classify Healthy and Diseased Cardiomyocytes by Contractility Profile.ACS Biomater Sci Eng. 2021 Jul 12;7(7):3043-3052. doi: 10.1021/acsbiomaterials.1c00418. Epub 2021 Jun 21. ACS Biomater Sci Eng. 2021. PMID: 34152732 Free PMC article.
-
Combinatorial Treatment of Human Cardiac Engineered Tissues With Biomimetic Cues Induces Functional Maturation as Revealed by Optical Mapping of Action Potentials and Calcium Transients.Front Physiol. 2020 Mar 12;11:165. doi: 10.3389/fphys.2020.00165. eCollection 2020. Front Physiol. 2020. PMID: 32226389 Free PMC article.
-
Patient-derived iPSC models of Friedreich ataxia: a new frontier for understanding disease mechanisms and therapeutic application.Transl Neurodegener. 2023 Sep 20;12(1):45. doi: 10.1186/s40035-023-00376-8. Transl Neurodegener. 2023. PMID: 37726850 Free PMC article. Review.
-
Arrhythmic Risk Assessment of Hypokalaemia Using Human Pluripotent Stem Cell-Derived Cardiac Anisotropic Sheets.Front Cell Dev Biol. 2021 Dec 6;9:681665. doi: 10.3389/fcell.2021.681665. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34938727 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

