Can novel early non-invasive biomarkers of embryo quality be identified with time-lapse imaging to predict live birth?
- PMID: 31287145
- PMCID: PMC6688874
- DOI: 10.1093/humrep/dez085
Can novel early non-invasive biomarkers of embryo quality be identified with time-lapse imaging to predict live birth?
Abstract
Study question: Can time-lapse imaging systems make it possible to identify novel early non-invasive biomarkers to predict live birth?
Summary answer: From mostly high-grade embryos, out of 35 morphometric, morphologic and morphokinetic variables, only pronuclei (PN) position at time of PN juxtaposition and the absence of multinucleated blastomeres at the 2-cell stage (MNB2cell), were potentially associated with live birth.
What is known already: Previous studies indicate that some kinetic markers may be predictive of blastocyst development and embryonic implantation. Certain teams have suggested including some of them in decisional algorithms for embryo transfers.
Study design, size, duration: Using a time-lapse incubator (EmbryoScope, Unisense FertiliTech), we retrospectively explored the associations between the morphometric, morphologic and morphokinetic parameters of oocytes, zygotes and embryos, and their associations with live birth. This study assessed 232 embryos from single embryo transfers after ICSI cycles performed between January 2014 and December 2017.
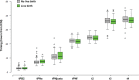
Participants/materials, setting, methods: The morphometric, morphologic and morphokinetic parameters (18, 4 and 13, respectively) of oocytes, zygotes and early embryos were studied retrospectively. The associations between these parameters were examined using a Spearman's correlation, Mann-Whitney or chi-squared test as appropriate. We examined whether these parameters were associated with outcomes in univariate and multivariate logistic regression analyses.
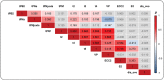
Main results and the role of chance: Central PN juxtaposition was associated with a 2-fold increase in the odds of live birth (OR = 2.20; 95% CI, [1.26-3.89]; P = 0.006), while the presence of MNB2cell was associated with half the odds of live birth (OR = 0.51; 95% CI, [0.27-0.95]; P = 0.035). These two parameters were independent of embryo kinetics. The 33 remaining parameters had no significant association with the capacity of transferred embryos to develop to term.
Limitations, reasons for caution: Even though the population size was relatively small, our analyses were based on homogeneous cycles, i.e. young women whose transferred embryos were found to be high-grade according to conventional morphology evaluation. In addition, our conclusions were established from a specific, highly selected population, so other study populations, such as women in an older age bracket, may yield different results. Finally, because we assessed day 2/3 transfers, our findings cannot be generalized to embryos cultured up to the blastocyst stage.
Wider implications of the findings: It would be interesting to explore, prospectively, whether PN localisation is a relevant measure to predict embryo development when added into further algorithms and whether this parameter could be suitable for use in other IVF clinics. Further studies are needed, notably to explore the added value of timing evaluation in cohorts of embryos with low or intermediate morphology grade, as well as in other maternal populations (i.e. older women).
Study funding/competing interest(s): No external funding was used for this study. P. Sagot received funding from the following commercial companies: Merck Serono, Finox Biotech, Ferring, MSD France SAS, Teva Sante ́ SAS, Allergan France, Gedeon Richter France, Effik S.A., Karl Storz Endoscopie France, GE Medical Systems SCS, Laboratoires Genevrier, H.A.C. Pharma and Ipsen.All the authors confirm that none of this funding was used to support the research in this study. There are no patents, products in development or marketed products to declare. This does not alter the authors' adherence to all the journal policies on sharing data and materials.
Keywords: ICSI; embryo kinetics; embryo morphology; embryo morphometric parameters; live birth; time-lapse imaging system.
© The Author(s) 2019. Published by Oxford University Press.
Figures

Similar articles
-
In vitro maturation is associated with increased early embryo arrest without impairing morphokinetic development of useable embryos progressing to blastocysts.Hum Reprod. 2015 Aug;30(8):1842-9. doi: 10.1093/humrep/dev125. Epub 2015 Jun 3. Hum Reprod. 2015. PMID: 26040479
-
Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study.Hum Reprod. 2016 Oct;31(10):2245-54. doi: 10.1093/humrep/dew183. Epub 2016 Sep 2. Hum Reprod. 2016. PMID: 27591227
-
Timing of human preimplantation embryonic development is confounded by embryo origin.Hum Reprod. 2016 Feb;31(2):324-31. doi: 10.1093/humrep/dev296. Epub 2015 Dec 4. Hum Reprod. 2016. PMID: 26637491 Free PMC article.
-
Guidelines for the number of embryos to transfer following in vitro fertilization No. 182, September 2006.Int J Gynaecol Obstet. 2008 Aug;102(2):203-16. doi: 10.1016/j.ijgo.2008.01.007. Int J Gynaecol Obstet. 2008. PMID: 18773532 Review.
-
Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis.Hum Reprod. 2019 Mar 1;34(3):491-505. doi: 10.1093/humrep/dey388. Hum Reprod. 2019. PMID: 30689865
Cited by
-
Nucleation status of Day 2 pre-implantation embryos, acquired by time-lapse imaging during IVF, is associated with live birth.PLoS One. 2022 Sep 22;17(9):e0274502. doi: 10.1371/journal.pone.0274502. eCollection 2022. PLoS One. 2022. PMID: 36137104 Free PMC article.
-
Could cleaved embryo morphology and morphokinetics be associated with prenatal and neonatal outcomes?J Assist Reprod Genet. 2025 Jan;42(1):139-151. doi: 10.1007/s10815-024-03385-2. Epub 2025 Jan 9. J Assist Reprod Genet. 2025. PMID: 39786529
-
Noninvasive Biomarkers of Human Embryo Developmental Potential.Int J Mol Sci. 2025 May 21;26(10):4928. doi: 10.3390/ijms26104928. Int J Mol Sci. 2025. PMID: 40430065 Free PMC article. Review.
-
Detection of IL-6, IL-10, and TNF-α level in human single-blastocyst conditioned medium using ultrasensitive Single Molecule Array platform and its relationship with embryo quality and implantation: a pilot study.J Assist Reprod Genet. 2020 Jul;37(7):1695-1702. doi: 10.1007/s10815-020-01805-7. Epub 2020 May 15. J Assist Reprod Genet. 2020. PMID: 32415642 Free PMC article.
-
Association Between Human Embryo Culture Conditions, Cryopreservation, and the Potential Risk of Birth Defects in Children Conceived Through Assisted Reproduction Technology.Medicina (Kaunas). 2025 Jun 30;61(7):1194. doi: 10.3390/medicina61071194. Medicina (Kaunas). 2025. PMID: 40731824 Free PMC article. Review.
References
-
- Abeyta M, Behr B. Morphological assessment of embryo viability. Semin Reprod Med 2014;32:114–126. - PubMed
-
- Adamson GD, Abusief ME, Palao L, Witmer J, Palao LM, Gvakharia M. Improved implantation rates of day 3 embryo transfers with the use of an automated time-lapse–enabled test to aid in embryo selection. Fertil Steril 2016;105:369–375. - PubMed
-
- Aguilar J, Motato Y, Escribá MJ, Ojeda M, Muñoz E, Meseguer M. The human first cell cycle: impact on implantation. Reprod Biomed Online 2014;28:475–484. - PubMed
-
- Ahlstrom A, Park H, Bergh C, Selleskog U, Lundin K. Conventional morphology performs better than morphokinetics for prediction of live birth after day 2 transfer. Reprod Biomed Online 2016;33:61–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

