Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs
- PMID: 31287369
- PMCID: PMC6781258
- DOI: 10.1089/jamp.2018.1508
Devices for Improved Delivery of Nebulized Pharmaceutical Aerosols to the Lungs
Abstract
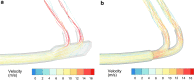
Nebulizers have a number of advantages for the delivery of inhaled pharmaceutical aerosols, including the use of aqueous formulations and the ability to deliver process-sensitive proteins, peptides, and biological medications. A frequent disadvantage of nebulized aerosols is poor lung delivery efficiency, which wastes valuable medications, increases delivery times, and may increase side effects of the medication. A focus of previous development efforts and previous nebulizer reviews, has been an improvement of the underlying nebulization technology controlling the breakup of a liquid into droplets. However, for a given nebulization technology, a wide range of secondary devices and strategies can be implemented to significantly improve lung delivery efficiency of the aerosol. This review focuses on secondary devices and technologies that can be implemented to improve the lung delivery efficiency of nebulized aerosols and potentially target the region of drug delivery within the lungs. These secondary devices may (1) modify the aerosol size distribution, (2) synchronize aerosol delivery with inhalation, (3) reduce system depositional losses at connection points, (4) improve the patient interface, or (5) guide patient inhalation. The development of these devices and technologies is also discussed, which often includes the use of computational fluid dynamic simulations, three-dimensional printing and rapid prototype device and airway model construction, realistic in vitro experiments, and in vivo analysis. Of the devices reviewed, the implementation of streamlined components may be the most direct and lowest cost approach to enhance aerosol delivery efficiency within nonambulatory nebulizer systems. For applications involving high-dose medications or precise dose administration, the inclusion of active devices to control aerosol size, guide inhalation, and synchronize delivery with inhalation hold considerable promise.
Keywords: inhalers; nebulizers; pharmaceutical aerosol devices; respiratory drug delivery.
Conflict of interest statement
Virginia Commonwealth University is currently pursuing patent protection of excipient enhanced growth aerosol delivery, aerosol generation devices, and patient interfaces, which if licensed, may provide a future financial interest to the authors.
Figures





Similar articles
-
Development of a High-Flow Nasal Cannula and Pharmaceutical Aerosol Combination Device.J Aerosol Med Pulm Drug Deliv. 2019 Aug;32(4):224-241. doi: 10.1089/jamp.2018.1488. Epub 2019 Mar 11. J Aerosol Med Pulm Drug Deliv. 2019. PMID: 30855199 Free PMC article.
-
Nebulizers for drug delivery to the lungs.Expert Opin Drug Deliv. 2015 Jun;12(6):889-900. doi: 10.1517/17425247.2015.995087. Epub 2014 Dec 23. Expert Opin Drug Deliv. 2015. PMID: 25534396 Review.
-
The function and performance of aqueous aerosol devices for inhalation therapy.J Pharm Pharmacol. 2016 May;68(5):556-78. doi: 10.1111/jphp.12541. Epub 2016 Apr 8. J Pharm Pharmacol. 2016. PMID: 27061412 Review.
-
Same lung deposited dose in dog dosing a fine and coarse aerosol indicates no difference in intranasal filtration.Int J Pharm. 2022 Aug 25;624:121977. doi: 10.1016/j.ijpharm.2022.121977. Epub 2022 Jul 2. Int J Pharm. 2022. PMID: 35792234
-
In Vitro Comparison of a Vibrating Mesh Nebulizer Operating in Inspiratory Synchronized and Continuous Nebulization Modes During Noninvasive Ventilation.J Aerosol Med Pulm Drug Deliv. 2016 Aug;29(4):328-36. doi: 10.1089/jamp.2015.1243. Epub 2016 Jun 16. J Aerosol Med Pulm Drug Deliv. 2016. PMID: 27310926
Cited by
-
Lipid-Based Inhalable Micro- and Nanocarriers of Active Agents for Treating Non-Small-Cell Lung Cancer.Pharmaceutics. 2023 May 10;15(5):1457. doi: 10.3390/pharmaceutics15051457. Pharmaceutics. 2023. PMID: 37242697 Free PMC article. Review.
-
Multiscale in silico lung modeling strategies for aerosol inhalation therapy and drug delivery.Curr Opin Biomed Eng. 2019 Sep;11:130-136. doi: 10.1016/j.cobme.2019.11.003. Epub 2019 Nov 13. Curr Opin Biomed Eng. 2019. PMID: 34642646 Free PMC article.
-
Comparison of the Application of Vibrating Mesh Nebulizer and Jet Nebulizer in Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-analysis.Int J Chron Obstruct Pulmon Dis. 2024 Mar 28;19:829-839. doi: 10.2147/COPD.S452191. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 38562440 Free PMC article.
-
Future Trends in Nebulized Therapies for Pulmonary Disease.J Pers Med. 2020 May 10;10(2):37. doi: 10.3390/jpm10020037. J Pers Med. 2020. PMID: 32397615 Free PMC article. Review.
-
Targeted Molecular Therapeutics for Pulmonary Diseases: Addressing the Need for Precise Drug Delivery.Handb Exp Pharmacol. 2024;284:313-328. doi: 10.1007/164_2023_703. Handb Exp Pharmacol. 2024. PMID: 38177399
References
-
- Boe J, Dennis J, O'Driscoll B, Bauer T, Carone M, Dautzenberg B, Diot P, Heslop K, and Lannefors L: European Respiratory Society Guidelines on the use of nebulizers: Guidelines prepared by a European Respiratory Society Task Force on the use of nebulizers. Eur Respir J. 2001;18:228–242 - PubMed
-
- Carvalho TC, and McConville JT: The function and performance of aqueous aerosol devices for inhalation therapy. J Pharm Pharmacol. 2016;68:556–578 - PubMed
-
- Martin AR, and Finlay WH: Nebulizers for drug delivery to the lungs. Expert Opin Drug Deliv. 2014;12:889–900 - PubMed
-
- Rubin BK: Pediatric aerosol therapy: New devices and new drugs. Respir Care. 2011;56:1411–1421 - PubMed
-
- Fink JB: Aerosol delivery to ventilated infant and pediatric patients. Respir Care. 2004;49:653–665 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

