PKCζ and JNK signaling regulate radiation-induced compensatory proliferation in parotid salivary glands
- PMID: 31287841
- PMCID: PMC6615637
- DOI: 10.1371/journal.pone.0219572
PKCζ and JNK signaling regulate radiation-induced compensatory proliferation in parotid salivary glands
Abstract
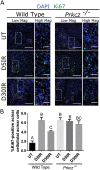
Radiotherapy is a common treatment option for head and neck cancer patients; however, the surrounding healthy salivary glands are often incidentally irradiated during the process. As a result, patients often experience persistent xerostomia and hyposalivation, which deceases their quality of life. Clinically, there is currently no standard of care available to restore salivary function. Repair of epithelial wounds involves cellular proliferation and establishment of polarity in order to regenerate the tissue. This process is partially mediated by protein kinase C zeta (PKCζ), an apical polarity regulator; however, its role following radiation damage is not completely understood. Using an in vivo radiation model, we show a significant decrease in active PKCζ in irradiated murine parotid glands, which correlates with increased proliferation that is sustained through 30 days post-irradiation. Additionally, salivary glands in PKCζ null mice show increased basal proliferation which radiation treatment did not further potentiate. Radiation damage also activates Jun N-terminal kinase (JNK), a proliferation-inducing mitogen-activated protein kinase normally inhibited by PKCζ. In both a PKCζ null mouse model and in primary salivary gland cell cultures treated with a PKCζ inhibitor, there was increased JNK activity and production of downstream proliferative transcripts. Collectively, these findings provide a potential molecular link by which PKCζ suppression following radiation damage promotes JNK activation and radiation-induced compensatory proliferation in the salivary gland.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
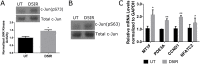


Similar articles
-
Indomethacin Treatment Post-irradiation Improves Mouse Parotid Salivary Gland Function via Modulation of Prostaglandin E2 Signaling.Front Bioeng Biotechnol. 2021 Jul 21;9:697671. doi: 10.3389/fbioe.2021.697671. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34381764 Free PMC article.
-
Dietary nitrate supplementation prevents radiotherapy-induced xerostomia.Elife. 2021 Sep 28;10:e70710. doi: 10.7554/eLife.70710. Elife. 2021. PMID: 34581269 Free PMC article.
-
aPKCζ-dependent Repression of Yap is Necessary for Functional Restoration of Irradiated Salivary Glands with IGF-1.Sci Rep. 2018 Apr 20;8(1):6347. doi: 10.1038/s41598-018-24678-4. Sci Rep. 2018. PMID: 29679075 Free PMC article.
-
Partial irradiation of the parotid gland.Semin Radiat Oncol. 2001 Jul;11(3):234-9. doi: 10.1053/srao.2001.23484. Semin Radiat Oncol. 2001. PMID: 11447580 Review.
-
Pathophysiology and management of radiation-induced xerostomia.J Support Oncol. 2005 May-Jun;3(3):191-200. J Support Oncol. 2005. PMID: 15915820 Review.
Cited by
-
Cell Death in the Tumor Microenvironment: Implications for Cancer Immunotherapy.Cells. 2020 Sep 29;9(10):2207. doi: 10.3390/cells9102207. Cells. 2020. PMID: 33003477 Free PMC article. Review.
-
Indomethacin Treatment Post-irradiation Improves Mouse Parotid Salivary Gland Function via Modulation of Prostaglandin E2 Signaling.Front Bioeng Biotechnol. 2021 Jul 21;9:697671. doi: 10.3389/fbioe.2021.697671. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34381764 Free PMC article.
-
Autologous mesenchymal stem cells offer a new paradigm for salivary gland regeneration.Int J Oral Sci. 2023 May 10;15(1):18. doi: 10.1038/s41368-023-00224-5. Int J Oral Sci. 2023. PMID: 37165024 Free PMC article. Review.
-
Radiation-induced changes in energy metabolism result in mitochondrial dysfunction in salivary glands.Sci Rep. 2024 Jan 8;14(1):845. doi: 10.1038/s41598-023-50877-9. Sci Rep. 2024. PMID: 38191641 Free PMC article.
-
900 MHz Radiofrequency Field Induces Mitochondrial Unfolded Protein Response in Mouse Bone Marrow Stem Cells.Front Public Health. 2021 Aug 26;9:724239. doi: 10.3389/fpubh.2021.724239. eCollection 2021. Front Public Health. 2021. PMID: 34513791 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

