Genomic and Transcriptomic Determinants of Therapy Resistance and Immune Landscape Evolution during Anti-EGFR Treatment in Colorectal Cancer
- PMID: 31287991
- PMCID: PMC6617392
- DOI: 10.1016/j.ccell.2019.05.013
Genomic and Transcriptomic Determinants of Therapy Resistance and Immune Landscape Evolution during Anti-EGFR Treatment in Colorectal Cancer
Abstract
Despite biomarker stratification, the anti-EGFR antibody cetuximab is only effective against a subgroup of colorectal cancers (CRCs). This genomic and transcriptomic analysis of the cetuximab resistance landscape in 35 RAS wild-type CRCs identified associations of NF1 and non-canonical RAS/RAF aberrations with primary resistance and validated transcriptomic CRC subtypes as non-genetic predictors of benefit. Sixty-four percent of biopsies with acquired resistance harbored no genetic resistance drivers. Most of these had switched from a cetuximab-sensitive transcriptomic subtype at baseline to a fibroblast- and growth factor-rich subtype at progression. Fibroblast-supernatant conferred cetuximab resistance in vitro, confirming a major role for non-genetic resistance through stromal remodeling. Cetuximab treatment increased cytotoxic immune infiltrates and PD-L1 and LAG3 immune checkpoint expression, potentially providing opportunities to treat cetuximab-resistant CRCs with immunotherapy.
Keywords: EGFR; cancer evolution; cancer genomics; cancer-associated fibroblasts; cetuximab; colorectal cancer; drug resistance mechanisms; immunotherapy; molecular subtype; predictive biomarker.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
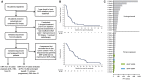




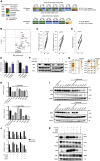

Comment in
-
The Stromal and Immune Landscape of Colorectal Cancer Progression during Anti-EGFR Therapy.Cancer Cell. 2019 Jul 8;36(1):1-3. doi: 10.1016/j.ccell.2019.06.001. Cancer Cell. 2019. PMID: 31287988
-
Microenvironment plasticity confers cetuximab resistance.Nat Rev Clin Oncol. 2019 Sep;16(9):527. doi: 10.1038/s41571-019-0259-4. Nat Rev Clin Oncol. 2019. PMID: 31324873 No abstract available.
References
-
- Allegra C.J., Rumble R.B., Hamilton S.R., Mangu P.B., Roach N., Hantel A., Schilsky R.L. Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J. Clin. Oncol. 2016;34:179–185. - PubMed
- Allegra, C.J., Rumble, R.B., Hamilton, S.R., Mangu, P.B., Roach, N., Hantel, A., and Schilsky, R.L.. (2016). Extended RAS gene mutation testing in metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update 2015. J. Clin. Oncol. 34, 179-185. - PubMed
-
- Amado R.G., Wolf M., Peeters M., Van Cutsem E., Siena S., Freeman D.J., Juan T., Sikorski R., Suggs S., Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:1626–1634. - PubMed
- Amado, R.G., Wolf, M., Peeters, M., Van Cutsem, E., Siena, S., Freeman, D.J., Juan, T., Sikorski, R., Suggs, S., Radinsky, R., et al. (2008). Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 1626-1634. - PubMed
-
- Angelova M., Mlecnik B., Vasaturo A., Bindea G., Fredriksen T., Lafontaine L., Buttard B., Morgand E., Bruni D., Jouret-Mourin A. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765.e16. - PubMed
- Angelova, M., Mlecnik, B., Vasaturo, A., Bindea, G., Fredriksen, T., Lafontaine, L., Buttard, B., Morgand, E., Bruni, D., Jouret-Mourin, A., et al. (2018). Evolution of metastases in space and time under immune selection. Cell 175, 751-765.e16. - PubMed
-
- Arena S., Bellosillo B., Siravegna G., Martinez A., Canadas I., Lazzari L., Ferruz N., Russo M., Misale S., Gonzalez I. Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin. Cancer Res. 2015;21:2157–2166. - PubMed
- Arena, S., Bellosillo, B., Siravegna, G., Martinez, A., Canadas, I., Lazzari, L., Ferruz, N., Russo, M., Misale, S., Gonzalez, I., et al. (2015). Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin. Cancer Res. 21, 2157-2166. - PubMed
-
- Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R., Albright A., Cheng J.D., Kang S.P., Shankaran V. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017;127:2930–2940. - PMC - PubMed
- Ayers, M., Lunceford, J., Nebozhyn, M., Murphy, E., Loboda, A., Kaufman, D.R., Albright, A., Cheng, J.D., Kang, S.P., Shankaran, V., et al. (2017). IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930-2940. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

