Liver-Specific, but Not Retina-Specific, Hepcidin Knockout Causes Retinal Iron Accumulation and Degeneration
- PMID: 31287995
- PMCID: PMC6723216
- DOI: 10.1016/j.ajpath.2019.05.022
Liver-Specific, but Not Retina-Specific, Hepcidin Knockout Causes Retinal Iron Accumulation and Degeneration
Abstract
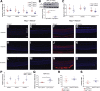
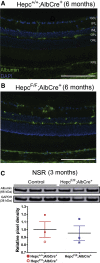
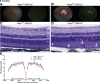
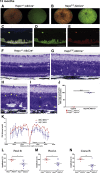
The liver secretes hepcidin (Hepc) into the bloodstream to reduce blood iron levels. Hepc accomplishes this by triggering degradation of the only known cellular iron exporter ferroportin in the gut, macrophages, and liver. We previously demonstrated that systemic Hepc knockout (HepcKO) mice, which have high serum iron, develop retinal iron overload and degeneration. However, it was unclear whether this is caused by high blood iron levels or, alternatively, retinal iron influx that would normally be regulated by retina-produced Hepc. To address this question, retinas of liver-specific and retina-specific HepcKO mice were studied. Liver-specific HepcKO mice had elevated blood and retinal pigment epithelium (RPE) iron levels and increased free (labile) iron levels in the retina, despite an intact blood-retinal barrier. This led to RPE hypertrophy associated with lipofuscin-laden lysosome accumulation. Photoreceptors also degenerated focally. In contrast, there was no change in retinal or RPE iron levels or degeneration in the retina-specific HepcKO mice. These data indicate that high blood iron levels can lead to retinal iron accumulation and degeneration. High blood iron levels can occur in patients with hereditary hemochromatosis or result from use of iron supplements or multiple blood transfusions. Our results suggest that high blood iron levels may cause or exacerbate retinal disease.
Copyright © 2019 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
The oral iron chelator deferiprone protects against systemic iron overload-induced retinal degeneration in hepcidin knockout mice.Invest Ophthalmol Vis Sci. 2014 Jun 26;55(7):4525-32. doi: 10.1167/iovs.14-14568. Invest Ophthalmol Vis Sci. 2014. PMID: 24970260 Free PMC article.
-
Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice.Invest Ophthalmol Vis Sci. 2011 Jan 5;52(1):109-18. doi: 10.1167/iovs.10-6113. Invest Ophthalmol Vis Sci. 2011. PMID: 20811044 Free PMC article.
-
Iron Accumulates in Retinal Vascular Endothelial Cells But Has Minimal Retinal Penetration After IP Iron Dextran Injection in Mice.Invest Ophthalmol Vis Sci. 2019 Oct 1;60(13):4378-4387. doi: 10.1167/iovs.19-28250. Invest Ophthalmol Vis Sci. 2019. PMID: 31634395 Free PMC article.
-
Expression and function of iron-regulatory proteins in retina.IUBMB Life. 2010 May;62(5):363-70. doi: 10.1002/iub.326. IUBMB Life. 2010. PMID: 20408179 Free PMC article. Review.
-
[Retinal degeneration in patients with hereditary ceruloplasmin deficiency].Nihon Naika Gakkai Zasshi. 2004 Aug 10;93(8):1591-6. doi: 10.2169/naika.93.1591. Nihon Naika Gakkai Zasshi. 2004. PMID: 15384688 Review. Japanese. No abstract available.
Cited by
-
A vicious cycle of bisretinoid formation and oxidation relevant to recessive Stargardt disease.J Biol Chem. 2021 Jan-Jun;296:100259. doi: 10.1016/j.jbc.2021.100259. Epub 2021 Jan 7. J Biol Chem. 2021. PMID: 33837742 Free PMC article.
-
Cytochrome P450 1B1 Expression Regulates Intracellular Iron Levels and Oxidative Stress in the Retinal Endothelium.Int J Mol Sci. 2023 Jan 26;24(3):2420. doi: 10.3390/ijms24032420. Int J Mol Sci. 2023. PMID: 36768740 Free PMC article.
-
Insights into the liver-eyes connections, from epidemiological, mechanical studies to clinical translation.J Transl Med. 2023 Oct 10;21(1):712. doi: 10.1186/s12967-023-04543-3. J Transl Med. 2023. PMID: 37817192 Free PMC article. Review.
-
From Rust to Quantum Biology: The Role of Iron in Retina Physiopathology.Cells. 2020 Mar 13;9(3):705. doi: 10.3390/cells9030705. Cells. 2020. PMID: 32183063 Free PMC article. Review.
-
Oxidative stress induces lysosomal membrane permeabilization and ceramide accumulation in retinal pigment epithelial cells.Dis Model Mech. 2023 Jul 1;16(7):dmm050066. doi: 10.1242/dmm.050066. Epub 2023 Jul 25. Dis Model Mech. 2023. PMID: 37401371 Free PMC article.
References
-
- Beard J.L. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S–579S. discussion 580S. - PubMed
-
- Aisen P., Enns C., Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int J Biochem Cell Biol. 2001;33:940–959. - PubMed
-
- Rogers B.S., Symons R.C.A., Komeima K., Shen J., Xiao W., Swaim M.E., Yuan Y.G., Kachi S., Campochiaro P.A. Differential sensitivity of cones to iron-mediated oxidative damage. Invest Ophthalmol Vis Sci. 2007;48:438–445. - PubMed
-
- Hahn P., Milam A.H., Dunaief J.L. Maculas affected by age-related macular degeneration contain increased chelatable iron in the retinal pigment epithelium and Bruch's membrane. Arch Ophthalmol. 2003;121:1099–1105. - PubMed
-
- Rouault T.A. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:551–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

