Candidalysin: discovery and function in Candida albicans infections
- PMID: 31288097
- PMCID: PMC6687503
- DOI: 10.1016/j.mib.2019.06.002
Candidalysin: discovery and function in Candida albicans infections
Abstract
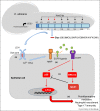
Candidalysin is a cytolytic peptide toxin secreted by the invasive form of the human pathogenic fungus, Candida albicans. Candidalysin is critical for mucosal and systemic infections and is a key driver of host cell activation, neutrophil recruitment and Type 17 immunity. Candidalysin is regarded as the first true classical virulence factor of C. albicans but also triggers protective immune responses. This review will discuss how candidalysin was discovered, the mechanisms by which this peptide toxin contributes to C. albicans infections, and how its discovery has advanced our understanding of fungal pathogenesis and disease.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci Transl Med. 2012;4 - PubMed
-
- Brown G.D., Denning D.W., Levitz S.M. Tackling human fungal infections. Science. 2012;336:647. - PubMed
-
- Netea M.G., Brown G.D. Fungal infections: the next challenge. Curr Opin Microbiol. 2012;15:403–405. - PubMed
-
- Gow N.A.R., Amin T., McArdle K., Brown A.J.P., Brown G.D., Warris A., The Wtsa-Mmfi C Strategic research funding: a success story for medical mycology. Trends Microbiol. 2018;10:811–813. - PubMed
-
- Moyes D.L., Wilson D., Richardson J.P., Mogavero S., Tang S.X., Wernecke J., Höfs S., Gratacap R.L., Robbins J., Runglall M. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 2016;532:64–68. - PMC - PubMed
-
Discovery of candidalysin, the first cytolytic peptide toxin identified in any human fungal pathogen.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

