Generation of Quiescent Cardiac Fibroblasts From Human Induced Pluripotent Stem Cells for In Vitro Modeling of Cardiac Fibrosis
- PMID: 31288631
- PMCID: PMC6768436
- DOI: 10.1161/CIRCRESAHA.119.315491
Generation of Quiescent Cardiac Fibroblasts From Human Induced Pluripotent Stem Cells for In Vitro Modeling of Cardiac Fibrosis
Abstract
Rationale: Activated fibroblasts are the major cell type that secretes excessive extracellular matrix in response to injury, contributing to pathological fibrosis and leading to organ failure. Effective anti-fibrotic therapeutic solutions, however, are not available due to the poorly defined characteristics and unavailability of tissue-specific fibroblasts. Recent advances in single-cell RNA-sequencing fill such gaps of knowledge by enabling delineation of the developmental trajectories and identification of regulatory pathways of tissue-specific fibroblasts among different organs.
Objective: This study aims to define the transcriptome profiles of tissue-specific fibroblasts using recently reported mouse single-cell RNA-sequencing atlas and to develop a robust chemically defined protocol to derive cardiac fibroblasts (CFs) from human induced pluripotent stem cells for in vitro modeling of cardiac fibrosis and drug screening.
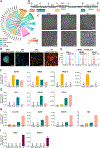
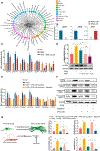
Methods and results: By analyzing the single-cell transcriptome profiles of fibroblasts from 10 selected mouse tissues, we identified distinct tissue-specific signature genes, including transcription factors that define the identities of fibroblasts in the heart, lungs, trachea, and bladder. We also determined that CFs in large are of the epicardial lineage. We thus developed a robust chemically defined protocol that generates CFs from human induced pluripotent stem cells. Functional studies confirmed that iPSC-derived CFs preserved a quiescent phenotype and highly resembled primary CFs at the transcriptional, cellular, and functional levels. We demonstrated that this cell-based platform is sensitive to both pro- and anti-fibrosis drugs. Finally, we showed that crosstalk between human induced pluripotent stem cell-derived cardiomyocytes and CFs via the atrial/brain natriuretic peptide-natriuretic peptide receptor-1 pathway is implicated in suppressing fibrogenesis.
Conclusions: This study uncovers unique gene signatures that define tissue-specific identities of fibroblasts. The bona fide quiescent CFs derived from human induced pluripotent stem cells can serve as a faithful in vitro platform to better understand the underlying mechanisms of cardiac fibrosis and to screen anti-fibrotic drugs.
Keywords: fibroblasts; fibrosis; induced pluripotent stem cells; transcriptome.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures





Comment in
-
Cardiac fibrosis research: two steps forward.Nat Rev Cardiol. 2019 Sep;16(9):515. doi: 10.1038/s41569-019-0245-7. Nat Rev Cardiol. 2019. PMID: 31341287 No abstract available.
References
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C and Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–63. - PubMed
-
- Paik DT, Tian L, Lee J, Sayed N, Chen IY, Rhee S, Rhee JW, Kim Y, Wirka RC, Buikema JW, Wu SM, Red-Horse K, Quertermous T and Wu JC. Large-scale single-cell RNA-seq reveals molecular signatures of heterogeneous populations of human induced pluripotent stem cell-derived endothelial cells. Circ Res. 2018;123:443–450. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

