The 'Pleasure&Pregnancy' web-based interactive educational programme versus expectant management in the treatment of unexplained subfertility: protocol for a randomised controlled trial
- PMID: 31289062
- PMCID: PMC6615847
- DOI: 10.1136/bmjopen-2018-025845
The 'Pleasure&Pregnancy' web-based interactive educational programme versus expectant management in the treatment of unexplained subfertility: protocol for a randomised controlled trial
Abstract
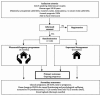
Introduction: Many subfertile couples are diagnosed with (relatively) unexplained subfertility and a good prognosis. National professional guidelines (eg, the Netherlands and UK) advise 'expectant management (EM)' for 6-12 months, in which no interaction with healthcare staff is offered. Underpowered studies indicate that face-to-face sex-counselling increases the ongoing pregnancy rates of these couples. In patients with other conditions, web-based interactive educational programmes have the same effect on sexual functioning as face-to-face sex counselling. The 'Pleasure&Pregnancy randomised controlled trial (RCT)' will examine in couples with unexplained subfertility and a good prognosis whether a new web-based interactive educational programme results in a higher chance of naturally conceiving an ongoing pregnancy within 6 months as compared with EM.
Methods and analysis: A multicentre RCT with cost-effectiveness analysis will include heterosexual couples diagnosed with (relatively) unexplained subfertility and a good prognosis in Dutch and Belgian secondary or tertiary fertility clinics. Couples will be randomised between 6 months of EM and 6 months of the Pleasure&Pregnancy-programme. This new web-based interactive educational programme includes eight progressive modules of information (on the biology of conception and pleasurable sex) and sensate focus, couple communication and mindfulness exercises. Couples are offered interaction with their coaches via email and can take part in three moderated chat sessions with peers. The primary outcome of this RCT is the probability of naturally conceiving an ongoing pregnancy within 6 months after randomisation. Secondary outcomes include time-to-pregnancy, live birth rate, costs, sexual functioning and personal and relational well-being. Analysis will be according to intention to treat.
Ethics and dissemination: This study has been approved by the Medical Ethical Committees of the Academic Medical Centre (the Netherlands) and the Leuven University Hospital (Belgium). The findings of this RCT will be disseminated through presentations at international scientific meetings and peer-reviewed publications.
Trail registration number: NTR5709; Pre-results.
Keywords: patient education; randomized controlled trial; sexuality; subfertility.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- van der Steeg JW, Steures P, Eijkemans MJ, et al. CECERM study group (Collaborative Effort for Clinical Evaluation in Reproductive Medicine). Pregnancy is predictable: a large-scale prospective external validation of the prediction of spontaneous pregnancy in subfertile couples. Hum Reprod 2007;22:536–42. - PubMed
-
- NVOG Richtlijn: Onverklaarde subfertiliteit: 2010-09-15, Versie: 1.0. https://www.nvog.nl/wp-content/uploads/2017/12/Onverklaarde-subfertilite... (Accessed 23 Oct 2018).
-
- Steures P, van der Steeg JW, Hompes PG, et al. Collaborative Effort on the Clinical Evaluation in Reproductive Medicine. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: a randomised clinical trial. Lancet 2006;368:216–21. - PubMed
-
- van Asselt K, Hinloopen RJ, Silvius AM, et al. Preconceptiezorg, wiens zorg eigenlijk? Huisarts Wet 2010;53:2–14. 10.1007/s12445-010-0002-y - DOI
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical